A STUDY on the REPRODUCTIVE SUCCESS of CAPTIVE WOLVERINES (Gulo G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MINNESOTA MUSTELIDS Young
By Blane Klemek MINNESOTA MUSTELIDS Young Naturalists the Slinky,Stinky Weasel family ave you ever heard anyone call somebody a weasel? If you have, then you might think Hthat being called a weasel is bad. But weasels are good hunters, and they are cunning, curious, strong, and fierce. Weasels and their relatives are mammals. They belong to the order Carnivora (meat eaters) and the family Mustelidae, also known as the weasel family or mustelids. Mustela means weasel in Latin. With 65 species, mustelids are the largest family of carnivores in the world. Eight mustelid species currently make their homes in Minnesota: short-tailed weasel, long-tailed weasel, least weasel, mink, American marten, OTTERS BY DANIEL J. COX fisher, river otter, and American badger. Minnesota Conservation Volunteer May–June 2003 n e MARY CLAY, DEMBINSKY t PHOTO ASSOCIATES r mammals a WEASELS flexible m Here are two TOM AND PAT LEESON specialized mustelid feet. b One is for climb- ou can recognize a ing and the other for hort-tailed weasels (Mustela erminea), long- The long-tailed weasel d most mustelids g digging. Can you tell tailed weasels (M. frenata), and least weasels eats the most varied e food of all weasels. It by their tubelike r which is which? (M. nivalis) live throughout Minnesota. In also lives in the widest Ybodies and their short Stheir northern range, including Minnesota, weasels variety of habitats and legs. Some, such as badgers, hunting. Otters and minks turn white in winter. In autumn, white hairs begin climates across North are heavy and chunky. Some, are excellent swimmers that hunt to replace their brown summer coat. -
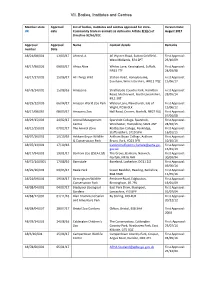
VII. Bodies, Institutes and Centres
VII. Bodies, Institutes and Centres Member state Approval List of bodies, institutes and centres approved for intra- Version Date: UK date Community trade in animals as defined in Article 2(1)(c) of August 2017 Directive 92/65/EEC Approval Approval Name Contact details Remarks number Date AB/21/08/001 13/03/17 Ahmed, A 46 Wyvern Road, Sutton Coldfield, First Approval: West Midlands, B74 2PT 23/10/09 AB/17/98/026 09/03/17 Africa Alive Whites Lane, Kessingland, Suffolk, First Approval: NR33 7TF 24/03/98 AB/17/17/005 15/06/17 All Things Wild Station Road, Honeybourne, First Approval: Evesham, Worcestershire, WR11 7QZ 15/06/17 AB/78/14/002 15/08/16 Amazonia Strathclyde Country Park, Hamilton First Approval: Road, Motherwell, North Lanarkshire, 28/05/14 ML1 3RT AB/29/12/003 06/04/17 Amazon World Zoo Park Watery Lane, Newchurch, Isle of First Approval: Wight, PO36 0LX 15/06/12 AB/17/08/065 08/03/17 Amazona Zoo Hall Road, Cromer, Norfolk, NR27 9JG First Approval: 07/04/08 AB/29/15/003 24/02/17 Animal Management Sparsholt College, Sparsholt, First Approval: Centre Winchester, Hampshire, SO21 2NF 24/02/15 AB/12/15/001 07/02/17 The Animal Zone Rodbaston College, Penkridge, First Approval: Staffordshire, ST19 5PH 16/01/15 AB/07/16/001 10/10/16 Askham Bryan Wildlife Askham Bryan College, Askham First Approval: & Conservation Park Bryan, York, YO23 3FR 10/10/16 AB/07/13/001 17/10/16 [email protected]. First Approval: gov.uk 15/01/13 AB/17/94/001 19/01/17 Banham Zoo (ZSEA Ltd) The Grove, Banham, Norwich, First Approval: Norfolk, NR16 -

Verzeichnis Der Europäischen Zoos Arten-, Natur- Und Tierschutzorganisationen
uantum Q Verzeichnis 2021 Verzeichnis der europäischen Zoos Arten-, Natur- und Tierschutzorganisationen Directory of European zoos and conservation orientated organisations ISBN: 978-3-86523-283-0 in Zusammenarbeit mit: Verband der Zoologischen Gärten e.V. Deutsche Tierpark-Gesellschaft e.V. Deutscher Wildgehege-Verband e.V. zooschweiz zoosuisse Schüling Verlag Falkenhorst 2 – 48155 Münster – Germany [email protected] www.tiergarten.com/quantum 1 DAN-INJECT Smith GmbH Special Vet. Instruments · Spezial Vet. Geräte Celler Str. 2 · 29664 Walsrode Telefon: 05161 4813192 Telefax: 05161 74574 E-Mail: [email protected] Website: www.daninject-smith.de Verkauf, Beratung und Service für Ferninjektionsgeräte und Zubehör & I N T E R Z O O Service + Logistik GmbH Tranquilizing Equipment Zootiertransporte (Straße, Luft und See), KistenbauBeratung, entsprechend Verkauf undden Service internationalen für Ferninjektionsgeräte und Zubehör Vorschriften, Unterstützung bei der Beschaffung der erforderlichenZootiertransporte Dokumente, (Straße, Vermittlung Luft und von See), Tieren Kistenbau entsprechend den internationalen Vorschriften, Unterstützung bei der Beschaffung der Celler Str.erforderlichen 2, 29664 Walsrode Dokumente, Vermittlung von Tieren Tel.: 05161 – 4813192 Fax: 05161 74574 E-Mail: [email protected] Str. 2, 29664 Walsrode www.interzoo.deTel.: 05161 – 4813192 Fax: 05161 – 74574 2 e-mail: [email protected] & [email protected] http://www.interzoo.de http://www.daninject-smith.de Vorwort Früheren Auflagen des Quantum Verzeichnis lag eine CD-Rom mit der Druckdatei im PDF-Format bei, welche sich großer Beliebtheit erfreute. Nicht zuletzt aus ökologischen Gründen verzichten wir zukünftig auf eine CD-Rom. Stattdessen kann das Quantum Verzeichnis in digitaler Form über unseren Webshop (www.buchkurier.de) kostenlos heruntergeladen werden. Die Datei darf gerne kopiert und weitergegeben werden. -

Amtliche Veterinärnachrichten Des Bundesministeriums Für Gesundheit Und Frauen V E T E R I N Ä R V E R W a L T U N G
REPUBLIK ÖSTERREICH Republic of Austria - République d’Autriche Amtliche Veterinärnachrichten des Bundesministeriums für Gesundheit und Frauen V e t e r i n ä r v e r w a l t u n g Official Veterinary Bulletin - Bulletin Vétérinaire Officiel Nr. 3/März 2006 Wien, am 25. April 2006 79. Jahrgang T h e m e n ü b e r s i c h t 1. Tierseuchen 2. Verlautbarung gemäß § 13 Abs. 3 EBVO 2001, BGBl. II Nr. 355/2001 der von der EG zugelassenen Drittlandbetriebe für: Milch und Milcherzeugnisse, Fischereierzeugnisse, Ernte- gebiete für lebende Muscheln, Stachelhäuter, Manteltiere und Meeresschnecken sowie Reinigungs- und Versandbetriebe für lebende Muscheln GZ 74.210/0003-IV/B/8/2006 3. Verlautbarung von Schlachthäusern, Zerlegungsbetrieben, Kühlhäusern und Verarbeitungsbetrieben gemäß § 13 Abs. 2 EBVO 2001, BGBl. II Nr. 355/2001 GZ 74.210/0004-IV/B/8/2006 4. Liste der zum innergemeinschaftlichen Handel zugelassenen Fleischbetriebe in Österreich GZ 74.410/0023-IV/B/7/2006 DVR: 21092541 P.b.b. Erscheinungsort Wien, Verlagspostamt 1030 Wien 5. Listen der zum innergemeinschaftlichen Handel zugelassenen Einrichtungen, Institute und Zentren in Österreich entsprechend RL 92/65/EWG i.d.l.g.F. GZ 74.420/0024-IV/B/8/2006 6. Liste der zugelassenen Betriebe für tierische Nebenprodukte in Österreich gemäß Verordnung (EG) Nr. 1774/2002 GZ 74.410/0028-IV/B/7/2006 7. Veröffentlichungen auf Grund § 6 Abs. 2, § 10 Abs. 2, § 13 Abs. 2 und 3, § 16 Abs. 1, 2 und 3, § 27 Abs. 3, § 33 Abs. 2, § 34 Abs. 1 und 3 und § 39 Abs. -

California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group
California Wildlife Habitat Relationships System California Department of Fish and Wildlife California Interagency Wildlife Task Group WOLVERINE Gulo gulo Family: MUSTELIDAE Order: CARNIVORA Class: MAMMALIA M159 Written by: V. Johnson Reviewed by: H. Shellhammer Edited by: J. Harris, R. Duke DISTRIBUTION, ABUNDANCE, AND SEASONALITY A scarce resident of North Coast mountains and Sierra Nevada. Sightings range from Del Norte and Trinity cos. east through Siskiyou and Shasta cos., and south through Tulare Co. A few possible sightings occur in the north coastal region as far south as Lake Co. Habitat distribution in California is poorly known for the North Coast and northern Sierra Nevada. In north coastal areas, has been observed in Douglas-fir and mixed conifer habitats, and probably uses red fir, lodgepole, wet meadow, and montane riparian habitats. Most sightings in this region range from 500-1500 m (1600-4800 ft). In the northern Sierra Nevada, have been found in mixed conifer, red fir, and lodgepole habitats, and probably use subalpine conifer, alpine dwarf-shrub, wet meadow, and montane riparian habitats. Elevations in the northern Sierra Nevada mostly fall in the range of 1300-2300 m (4300-7300 ft). Habitats used in the southern Sierra Nevada include red fir, mixed conifer, lodgepole, subalpine conifer, alpine dwarf-shrub, barren, and probably wet meadows, montane chaparral, and Jeffrey pine. Elevations in the southern Sierra Nevada mostly are from 2000-3400 m (6400-10,800 ft). May travel extensively. There are indications that wolverines may be increasing in California (Grinnell et al. 1937, Ingles 1965, Yocom 1973, 1974, Johnson 1977, Schempf and White 1977, California Department of Fish and Game 1980a). -

ATIC0943 {By Email}
Animal and Plant Health Agency T 0208 2257636 Access to Information Team F 01932 357608 Weybourne Building Ground Floor Woodham Lane www.gov.uk/apha New Haw Addlestone Surrey KT15 3NB Our Ref: ATIC0943 {By Email} 4 October 2016 Dear PROVISION OF REQUESTED INFORMATION Thank you for your request for information about zoos which we received on 26 September 2016. Your request has been handled under the Freedom of Information Act 2000. The information you requested and our response is detailed below: “Please can you provide me with a full list of the names of all Zoos in the UK. Under the classification of 'Zoos' I am including any place where a member of the public can visit or observe captive animals: zoological parks, centres or gardens; aquariums, oceanariums or aquatic attractions; wildlife centres; butterfly farms; petting farms or petting zoos. “Please also provide me the date of when each zoo has received its license under the Zoo License act 1981.” See Appendix 1 for a list that APHA hold on current licensed zoos affected by the Zoo License Act 1981 in Great Britain (England, Scotland and Wales), as at 26 September 2016 (date of request). The information relating to Northern Ireland is not held by APHA. Any potential information maybe held with the Department of Agriculture, Environment and Rural Affairs Northern Ireland (DAERA-NI). Where there are blanks on the zoo license start date that means the information you have requested is not held by APHA. Please note that the Local Authorities’ Trading Standard departments are responsible for administering and issuing zoo licensing under the Zoo Licensing Act 1981. -

Donating Hunted and Gathered Foods to ANMC
Donating hunted and gathered foods to ANMC You can donate hunted and gathered foods to the inpatient food service program at the Alaska Native Medical Center! Traditional foods are healing, nourishing medicine for our people and your donations will be greatly appreciated by our patients at ANMC. Donations we can accept: Donations we cannot accept: • Most wild game meat and bones (caribou, • Fox, any kind of bear, and walrus meat moose, deer, sheep, goat and beaver): must • Seal oil or whale oil (with or without meat) be whole, quartered, or roasts; meat cannot be ground • Fermented game meat (beaver tail, whale flipper, seal flipper, mikigaq, and walrus) • Most fish and seafood: must be gutted and gilled, with or without heads • Homemade canned or vacuum sealed foods • Marine mammal meat and fat (muktuk and • Smoked or dried seafood products (unless seal meat) those products are prepared in a seafood processing facility permitted under 18 AAC • Plants and berries: whole, fresh or frozen 34) • Fermented seafood products (salmon eggs, Donations will be accepted if: fish heads, and other) • The animal was not diseased • Molluscan shellfish • The animal was butchered, dressed, transported and stored to prevent cross Nothing will be wasted! contamination, undesired bacterial growth, • All donated items that are received will be or deteriorations; and the food would not used in their entirety. Any left over, trim cause significant health hazard or potential or items deemed “unservable” will be for human illness distributed to organizations that will utilize • The meat is whole, gutted, as quarters or as them in animal consumption, and waste will roasts without further processing go to local organizations that will use it (i.e. -

The Breeding in Captivity of the European Mink. Virgili Vidal, Albert
The breeding in captivity of the European mink (Mustela lutreola). Virgili Vidal, Albert. Final degree project. Faculty of Veterinary Medicine. June 2019. Objectives To know about the current situation of the European mink (Mustela lutreola), one of the most threatened mammals in Europe, from studies and data related to conservation, population (wild and captive) and causes of extinction. In addition, the research will focus on the effectiveness, results and difficulties of the recovery, conservation, breeding and release programs carried out in Europe, Spain and Catalonia. From this, some possible options will be evaluated to overcome the obstacles that the objective of saving the species is facing and if the programs applied nowadays are useful in the conservation of the European mink. European mink. Biology of the species The European mink is a little semi-aquatic carnivorous mammal of the Mustelidae family., known for it’s brown “chocolate” coat, one of the main reasons for it being hunted, and a white spot covering both lips. Its habitats include riparian forests and humid zones. It has solitary habits and a crepuscular and nocturnal activity in which it Figure 1: Geographic range of European mink. Historical distribution area (red), travels along the fluvial courses. Is a polygamous species, with a area where it is possibly still existent (purple) and confirmed existence area seasonal poliestrus and only breeds once a year giving birth to about (orange). Source: IUCN. three to six pups. In wildlife these animals live about 5-6 years. Conservation, breeding and recovery programs Situation of the species Many European countries (especially in Estonia, Germany, Spain and Until the end of XIX century it was very common and it was widely France) carry out reproduction and environment action programs in distributed around the whole continent, especially central and the wild. -

Eu Non-Native Organism Risk Assessment Scheme
EU NON-NATIVE SPECIES RISK ANALYSIS – RISK ASSESSMENT TEMPLATE V1.0 (8-06-16) EU NON-NATIVE ORGANISM RISK ASSESSMENT SCHEME Name of organism: Neovison vison Authors (alphabetical order): George Bouros, Jasja Dekker, Asun Gómez, Lauren A. Harrington, Zsolt Hegyeli, Calin Hodor, Kaarina Kauhala, Andreas Kranz, Erkki Korpimäki, Maurice La Haye, Xavier Lambin, David Macdonald, Sisco Mañas, Tiit Maran, Johan R. Michaux, Laura Moreno, Santiago Palazón, Madis Põdra, Pälvi Salo, Attila D. Sandor, Margarida Santos-Reis, Arnd Schreiber, Dan Traian Ionescu, Jiska van Dijk, Andrzej Zalewski, Iñigo Zuberogoitia Risk Assessment Area: European Union (28 Countries) Draft: 8/06/2016 1 EU NON-NATIVE SPECIES RISK ANALYSIS – RISK ASSESSMENT TEMPLATE V1.0 (8-06-16) EU CHAPEAU QUESTION RESPONSE 1. In how many EU member states has this species been recorded? List 24 countries: Austria, Belgium, Czech Republic, Denmark, Estonia, Finland, France, them. Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, United Kingdom. There is no American mink in Malta and information is not available from Bulgaria, Croatia and Cyprus (Macdonald & Harrington 2003, Bonesi & Palazón 2007, Dekker & Hofmeester 2014, Hegyeli & Kecskés 2014). In addition it is recorded in the non-member state Norway (The Norwegian Directorate for Nature Management 2011). American mink is kept also in fur farms almost all over Europe, in such countries as Germany, Denmark, Finland, Spain, Poland etc. (Kauhala 1996, Ruiz-Olmo et al 1997, Hammershoj et al 2005), though no longer in the United Kingdom. It is believed that keeping American mink as a pet is gaining popularity in some countries as well, for example in France (P. -

Museum of Natural History
p m r- r-' ME FYF-11 - - T r r.- 1. 4,6*. of the FLORIDA MUSEUM OF NATURAL HISTORY THE COMPARATIVE ECOLOGY OF BOBCAT, BLACK BEAR, AND FLORIDA PANTHER IN SOUTH FLORIDA David Steffen Maehr Volume 40, No. 1, pf 1-176 1997 == 46 1ms 34 i " 4 '· 0?1~ I. Al' Ai: *'%, R' I.' I / Em/-.Ail-%- .1/9" . -_____- UNIVERSITY OF FLORIDA GAINESVILLE Numbers of the BULLETIN OF THE FLORIDA MUSEUM OF NATURAL HISTORY am published at irregular intervals Volumes contain about 300 pages and are not necessarily completed in any one calendar year. JOHN F. EISENBERG, EDITOR RICHARD FRANZ CO-EDIWR RHODA J. BRYANT, A£ANAGING EMOR Communications concerning purchase or exchange of the publications and all manuscripts should be addressed to: Managing Editor. Bulletin; Florida Museum of Natural Histoty, University of Florida P. O. Box 117800, Gainesville FL 32611-7800; US.A This journal is printed on recycled paper. ISSN: 0071-6154 CODEN: BF 5BAS Publication date: October 1, 1997 Price: $ 10.00 Frontispiece: Female Florida panther #32 treed by hounds in a laurel oak at the site of her first capture on the Florida Panther National Wildlife Refuge in central Collier County, 3 February 1989. Photograph by David S. Maehr. THE COMPARATIVE ECOLOGY OF BOBCAT, BLACK BEAR, AND FLORIDA PANTHER IN SOUTH FLORIDA David Steffen Maehri ABSTRACT Comparisons of food habits, habitat use, and movements revealed a low probability for competitive interactions among bobcat (Lynx ndia). Florida panther (Puma concotor cooi 1 and black bear (Urns amencanus) in South Florida. All three species preferred upland forests but ©onsumed different foods and utilized the landscape in ways that resulted in ecological separation. -

The 2008 IUCN Red Listings of the World's Small Carnivores
The 2008 IUCN red listings of the world’s small carnivores Jan SCHIPPER¹*, Michael HOFFMANN¹, J. W. DUCKWORTH² and James CONROY³ Abstract The global conservation status of all the world’s mammals was assessed for the 2008 IUCN Red List. Of the 165 species of small carni- vores recognised during the process, two are Extinct (EX), one is Critically Endangered (CR), ten are Endangered (EN), 22 Vulnerable (VU), ten Near Threatened (NT), 15 Data Deficient (DD) and 105 Least Concern. Thus, 22% of the species for which a category was assigned other than DD were assessed as threatened (i.e. CR, EN or VU), as against 25% for mammals as a whole. Among otters, seven (58%) of the 12 species for which a category was assigned were identified as threatened. This reflects their attachment to rivers and other waterbodies, and heavy trade-driven hunting. The IUCN Red List species accounts are living documents to be updated annually, and further information to refine listings is welcome. Keywords: conservation status, Critically Endangered, Data Deficient, Endangered, Extinct, global threat listing, Least Concern, Near Threatened, Vulnerable Introduction dae (skunks and stink-badgers; 12), Mustelidae (weasels, martens, otters, badgers and allies; 59), Nandiniidae (African Palm-civet The IUCN Red List of Threatened Species is the most authorita- Nandinia binotata; one), Prionodontidae ([Asian] linsangs; two), tive resource currently available on the conservation status of the Procyonidae (raccoons, coatis and allies; 14), and Viverridae (civ- world’s biodiversity. In recent years, the overall number of spe- ets, including oyans [= ‘African linsangs’]; 33). The data reported cies included on the IUCN Red List has grown rapidly, largely as on herein are freely and publicly available via the 2008 IUCN Red a result of ongoing global assessment initiatives that have helped List website (www.iucnredlist.org/mammals). -

Monitoring of Forest Mammals Listed in the Annexes IV and V of the Habitat Directive (Except Bats)
Monitoring biodiversity in Luxembourg: what is left to be done? 22 November 2017 Monitoring of forest mammals listed in the Annexes IV and V of the Habitat Directive (except bats) Distribution and evolution since 2010: Marc Moes GEODATA • Hazel dormouse, • Wildcat, Xavier Mestdagh • Pine marten, Lionel L’Hoste • Polecat. LIST Hazel Dormouse Muscardinus avellanarius 2 Hazel Dormouse Muscardinus avellanarius Methodology • Identification of “potentially” suitable habitats • Random and stratified sampling design 90 squares (1x1 Km) selected Looking for “summer” nest in 4 “favourable” sites/square during Oct/Nov Mainly brambles, shrub covered area and forest edge • Triennal sampling procedure 3 Hazel Dormouse Muscardinus avellanarius Results • Since 2010: 102 squares were surveyed • 2017 sampling is still in progress • From 2013 to 2015: repeated survey for some squares 1st passage: 1124 sites investigated so far! 4 Hazel Dormouse Muscardinus avellanarius Results: Sites level (1st passage) 1st passage 2010-2012 2013-2015 2016-2018 Number of sites 596 336 192 surveyed ( + + ) = 1124 sites Number of favourable sites 294 269 149 surveyed Number of favourable sites 108 169 83 with proof of presence Average= 51% 5 Hazel Dormouse Muscardinus avellanarius Results: Squares level - analysis based on the 1st passage 1st passage 2010-2012 2013-2015 2016-2018 2010/ 2013/ 2012 2015 Number of squares ✔ ✔ surveyed with at least one 96 78 28 favourable site ✔ ✘ Number of squares ✘ ✔ surveyed with at least one ✘ ✘ favourable site 70 66 26 AND with proof