Designs That Lacked Inherent Safety: Case Histories
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barton-Upon-Humber Town Council
BARTON-UPON-HUMBER TOWN COUNCIL Shirley Richards Town Clerk Council Office Assembly Rooms Queen Street BARTON-UPON-HUMBER Telephone: 01652 633598 North Lincolnshire email:[email protected] DN18 5QP www.barton-upon-humber.org.uk/barton town council Our Ref: SAR/CMC/AGENDA 20 June 2017 Dear Sir/Madam Notice is given that a PLANNING COMMITTEE MEETING of BARTON-UPON-HUMBER TOWN COUNCIL to be held in THE COMMITTEE ROOM, THE ASSEMBLY ROOMS, QUEEN STREET, BARTON-UPON-HUMBER on MONDAY 26 JUNE 2017 COMMENCING at 7.00 p.m. The press and public are welcome to attend. Yours faithfully Shirley Richards Shirley Richards Town Clerk AGENDA 1. Apologies for absence, if any. 2. Declarations of Interest: (a) To record declarations of interest by any member of the council in respect of the agenda items listed below. Members declaring interests should identify the agenda item and type of interest being declared. (b) To note dispensations given to any member of the council in respect of the agenda items listed below. 3. Planning matters: (a) To consider making comments, if any, on the following Planning Applications to North Lincolnshire Council: (1) PA/2017/826 - Mr G Nettleton – Planning permission for change of use from barn to residential dwelling at Little Grange Farm, Ferriby Road, Barton; -2- (2) PA/2017/765 – Community Partners Ltd (Mr Steve Green) – Advertisement consent to display signs on roundabouts to include the following locations: A15/A1077 Ferriby Road Barton Interchange, A1077 Ferriby Road/Forkedale RAB, A15/M180 Elsham, A18 Bigby -

BGS Report, Single Column Layout
Mineral Resource Information in Support of National, Regional and Local Planning Humberside (comprising East Riding of Yorkshire, North Lincolnshire, North East Lincolnshire and City of Kingston upon Hull). Commissioned Report CR/04/227N BRITISH GEOLOGICAL SURVEY COMMISSIONED REPORT CR/04/227N Mineral Resource Information in Support of National, Regional and Local Planning Humberside (comprising East Riding of Yorkshire, North Lincolnshire, North east Lincolnshire and City of Kingston upon Hull) D J Harrison, F M McEvoy, P J Henney, D G Cameron, E J Steadman, S F Hobbs, N A Spencer, D J Evans, G K Lott, E M Bartlett, M H Shaw, D E Highley and T B Colman The National Grid and other Ordnance Survey data are used This report accompanies the 1:100 000 scale map: Humberside with the permission of the Mineral Resources Controller of Her Majesty’s Stationery Office. Licence No: 100017897/2005. Keywords Mineral resources, mineral planning, East Yorkshire and Humberside. Front cover Excavator working bed of sand from recent Blown Sand (Recent) at Cove Farm Quarry near Haxey. Bibliographical reference HARRISON, D J, and 12 others, 2005. Mineral Resource Information in Support of National, Regional and Local Planning - East Yorkshire and Humberside. British Geological Survey Commissioned Report, CR/04/227N. 18pp © Crown Copyright 2005. Keyworth, Nottingham British Geological Survey 2005 BRITISH GEOLOGICAL SURVEY The full range of Survey publications is available from the BGS British Geological Survey offices Sales Desks at Nottingham, Edinburgh and London; see contact details below or shop online at www.geologyshop.com Keyworth, Nottingham NG12 5GG The London Information Office also maintains a reference 0115-936 3241 Fax 0115-936 3488 collection of BGS publications including maps for consultation. -

Land at Flixborough & Gunness, Letting Particulars.Cdr
FLIXBOROUGH & GUNNESS NORTH LINCOLNSHIRE (Scunthorpe 3 miles, Humber Bridge 19 miles) 498.06 ACRES (201.56 Hectares) or thereabouts HIGHLY PRODUCTIVE GRADE 1 AND 2 ARABLE LAND LOT 2 LOT 1 LOT 3 LOT 1 TO LET AS A WHOLE OR IN UP TO THREE LOTS BY INFORMAL TENDER LOTS 1 & 2: Six Year Farm Business Tenancy LOT 3: Two Year Farm Business Tenancy CLOSING DATE: 12 NOON MONDAY 15 MARCH 2021 Letting Agents DDM Agriculture Eastfield, Albert Street BRIGG, DN20 8HS Tel: 01652 653669 Fax: 01652 653311 DX: 24358 BRIGG Ref: Tori Email: [email protected] General Remarks and Stipulations Location The land is situated in the parishes of Flixborough and Gunness, with the fields comprising Lot 1 mainly located to the north east of Gunness and the fields comprising Lots 2 and 3 being situated on both sides of the A1077 Orbital Road. The town of Scunthorpe lies approximately three miles east of the village of Gunness, the city of Kingston upon Hull lies approximately thirty five miles to the north east. The Cathedral City of Lincoln lies approximately thirty three miles to the south. Please refer to the Site Plan on the back page of these particulars. Description The land comprises of the following: Lot 1 - 208.63Acres (84.43 Hectares) (shown Red on the Plan) – six adjoining arable fields which are regularly shaped and well suited to modern farming practices. Lot 2 - 153.65 Acres (62.18 Hectares) (shown Blue on the Plan) – seven adjoining arable fields, with two further parcels across Neap House Road, of regular shape and well suited to modern farming practices. -

SCOPING OPINION: Proposed North Lincolnshire Green Energy Park
SCOPING OPINION: Proposed North Lincolnshire Green Energy Park Case Reference: EN010116 Adopted by the Planning Inspectorate (on behalf of the Secretary of State) pursuant to Regulation 10 of The Infrastructure Planning (Environmental Impact Assessment) Regulations 2017 December 2020 [This page has been intentionally left blank] ii Scoping Opinion for North Lincolnshire Green Energy Park CONTENTS 1. INTRODUCTION ............................................................................ 1 1.1 Background .................................................................................... 1 1.2 The Planning Inspectorate’s Consultation............................................. 2 1.3 The European Union (Withdrawal Agreement) Act 2020 ......................... 3 2. THE PROPOSED DEVELOPMENT ..................................................... 4 2.1 Introduction ................................................................................... 4 2.2 Description of the Proposed Development ............................................ 4 2.3 The Planning Inspectorate’s Comments ............................................... 6 3. ES APPROACH............................................................................. 10 3.1 Introduction ................................................................................. 10 3.2 Relevant National Policy Statements (NPSs)....................................... 11 3.3 Scope of Assessment ..................................................................... 11 3.4 Coronavirus (COVID-19) Environmental -
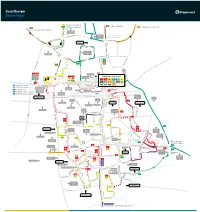
Scunthorpe Route Map
Scunthorpe Route Map 7 Continues as service 8 60 to Burton, Whitton 8 Continues as service 7 350 to Winterton, Barton, Hull 60 to Flixborough, Burton, Whitton Skippingdale Retail Park Ferry Road West Foxhills Outbound morning journeys Phoenix Parkway Industrial Estate Inbound evening journeys L Orbital Rd u rg n Mannabe Way e 8 b 7 u Crosby r g 7 8 W res N C a eedw l Portman Rd o p el y r a F S m er 8 ry CrosbyAv R n oa b d y W e Outwood R s d t Academy The Poplars Foxhills Foxhills Rd Warren Rd 8 Ferry Rd Rd Ferry 4 D 60 e w 8 350 Frodingham Rd s Winterton Rd 60 b u r y A A1077 Orbital Rd v UTC Brigg Rd Scotter Rd Lidl 1 1a Vivian Avenue Marsden Dv Sainsburys Gallagher Stanley Rd 1 1a Scunthorpe Town Centre Retail Park 7 Burn Rd St Lawrences Doncaster Rd 7 35 Academy Bus Station Tesco 1 1a 3 4 x4 7 7 90 60 HiltonAve 8 9 12 35 60 90 Doncaster Rd 360 361 399 350 35 to Amcotts, Crowle Doncaster Rd Cli Gardens d Moors Rd Mary St R d R 90 to Amcotts, Crowle North Lincolnshire r on e Kingsway ati Shopping Park v St to Crowle, Goole o Gardens 360 s Glanford Park 9 Hospital Scunthorpe Scunthorpe ol 361 to Westwoodside, Doncaster B United FC Minster Rd Church Lane Rowland Rd Kingsway 9 399 to Westwoodside, Doncaster Golf Course Midland Brumby Wood Lane Industrial Lodge Moor Brumby Wood Lane eck Rd Estate Steel Scotter Rd B Cottage Works A18 Kingsway The Brumby Pods 1 Wood Rd Ashby Cemetery Rd Quibell Park Brumby Frodingham Central Park UCNL Lilac A Crematorium ve Warwick Rd S a North n The Common Outward d Lindsey h College Academy o P 1a u lymouth -

Planning for Renewable Energy Development | Supplementary Planning Document | November 2011 Page | 1 Policy 7 - Community Impact
PlanningPlanning forfor RenewableRenewable EnergyEnergy DevelopmentDevelopment Spatial Planning Regeneration and Planning North Lincolnshire Council Supplementary Pittwood House Planning Document Ashby Road Scunthorpe November 2011 DN16 1AB Contents 1 Introduction ...........................................................................................................................................................................3 2 What is Renewable Energy? ...................................................................................................................................................5 3 North Lincolnshire & Renewable Energy ...............................................................................................................................7 On-Shore Wind ...................................................................................................................................................................7 Offshore Wind .....................................................................................................................................................................8 Carbon Capture and Storage ..................................................................................................................................................9 Biofuels ...............................................................................................................................................................................9 Biomass ............................................................................................................................................................................10 -

May 2021 Commencing at the Closure of the Annual General Meeting Which Commences at 7:00Pm at St Marks Church, Church Street, Amcotts
Amcotts Parish Council c/o The Clerk, Ms Clare Mason, The Sycamores, 21 Brewery Road, Crowle. North Lincolnshire, DN17 4LT. Telephone: 077257 23069 Email: [email protected] Dear Councillor, You are hereby summoned to a meeting of the FULL COUNCIL of Amcotts Parish Council, which will be held on Thursday 20th May 2021 commencing at the closure of the Annual General Meeting which commences at 7:00pm at St Marks Church, Church Street, Amcotts. Clare Mason Clerk to Amcotts Parish Council 15th May 2021 Members of the public are invited to attend the meeting and may speak on item(s) appearing on the Agenda, or on any other matter that falls within the Council’s terms of reference, at the discretion of the Chair. The public participation time is limited to 20 minutes at the beginning of the meeting. As the Council cannot legally reach a decision regarding matters not listed on this Agenda, matters may need to be added to future Agendas for later discussion and decision. 01/2021 Apologies To receive and approve any apologies for absence. 02/2021 Declarations of Interest To note any declarations of interest made by Councillors’ in respect of this Agenda (in accordance with the Council’s Code of Conduct and the Localism Act 2011). 03/2021 Minutes To approve the minutes of the Full Council meeting held on 4th March 2021 virtually 04/2021 Planning Applications – None but discussion may take place on Green Energy Park Flixborough KS3 Keadby development Granted PA2021/82. Linden Cottage, Trent Side, Amcotts granted to erect a 2 storey side -

North Lincolnshire Green Energy Park
North Lincolnshire Green Energy Park EIA Scoping Report October 2020 Project No.: 0483091 The business of sustainability NORTH LINCOLNSHIRE GREEN ENERGY PARK CONTENTS EIA Scoping Report CONTENTS 1. INTRODUCTION .......................................................................................................................... 1 1.1 Overview ........................................................................................................................................ 1 1.2 Requirement for EIA ...................................................................................................................... 1 1.3 The Applicant and EIA Team ......................................................................................................... 1 1.3.1 The Applicant ................................................................................................................ 1 1.3.2 The EIA Team ............................................................................................................... 2 1.3.3 The Contents of this Scoping Report ............................................................................ 3 2. POLICY AND LEGISLATIVE CONTEXT ..................................................................................... 4 2.1 Introduction .................................................................................................................................... 4 2.2 Article 50 of the Treaty on European Union .................................................................................. -

Enclosure & Agricultural Improvement in North-West Lincolnshire from Circa 1600 to 1850
ENCLOSURE & AGRICULTURAL IMPROVEMENT IN NORTH-WEST LINCOLNSHIRE FROM CIRCA 1600 TO 1850. Thomas M. Smith, MA Thesis submitted to the University of Nottingham for the degree of Doctor of Philosophy July 2012 i Abstract This study sets out to establish the link between enclosure and agricultural improvement in a group of parishes in north-west Lindsey, Lincolnshire between the sixteenth century and the mid-nineteenth century. In particular it emphasises the continuity of enclosure history through time, rather than concentrating only on the period of parliamentary enclosure as has often been the case in the past, and on links to agricultural improvement which include land reclamation, draining and warping. It shows that a simple explanation of enclosure in terms of driving up rents and allowing individual farmers to take their own farming decisions, fails to take into account the particular local circumstances of this area. Using a combination of enclosure documents and related material such as glebe terriers, land tax assessments, census materials, the 1801 agricultural returns and estate papers it sets out to show how agricultural improvement transformed both the landscape and the farming techniques in this area. In this process it covers a range of related topics including landownership, population, and the socio-economic structure of the villages of north-west Lindsey. It shows clearly that in this area enclosure is as much as anything associated with land drainage, and with improvements brought about by warping. These processes were interwoven, and separating enclosure out as a single movement underestimates the complexity of the farming arrangements required to ensure the most productive farming in this area. -

PDF Timetable 360
Revised: Mon 2 Sept 2019 Goole : Swinefleet : RSPB : Adlingfleet : Luddington : Crowle : Scunthorpe 360&361 Monday to Friday CD CH Saturday 360 360 361 361 361 361 360 361 361 361 361 Contact us... Goole North Street ............................0725 0725 0920 1120 1320 1520 1725 0820 1120 1430 1735 BusLine: 01482 59 29 29 Goole Boothferry Road Tesco .......... - - 0923 1123 1323 1523 - - - - - Old Goole............................................0728 0728 0931 1131 1331 1531 1728 0823 1123 1433 1738 eastyorkshirebuses.co.uk Swinefleet Church Lane ...................0733 0733 0936 1136 1336 1536 1733 0828 1128 1438 1743 Twitter @EYBuses Swinefleet Church Lane ...................0733 0733 0936 1136 1336 1536 1733 0828 1128 1438 1743 Reedness.............................................0738 0738 0941 1141 1341 1541 1738 0833 1133 1443 1748 Whitgift ...............................................0742 0742 0945 1145 1345 1545 1742 0837 1137 1447 1752 Ousefleet ............................................0744 0744 0947 1147 1347 1547 1744 0839 1139 1449 1754 No cash? RSPB Blacktoft Sands .......................0746 0746 0949 1149 1349 1549 1746 0841 1141 1451 1756 Adlingfleet ..........................................0749 0749 0952 1152 1352 1552 1749 0844 1144 1454 1759 No problem. Garthorpe ..........................................0753 0753 0956 1156 1356 1556 1753 0848 1148 1458 1803 Pay by contactless Luddington .........................................0757 0757 1000 1200 1400 1600 1757 0852 1152 1502 1807 Amcotts ..............................................0805 -

Beeches Barn Northfield Lane, Amcotts North Lincolnshire , DN17 4AH
Beeches Barn Northfield Lane, Amcotts North Lincolnshire , DN17 4AH Key Points Traditional two storey barn Currently used as garaging, workshop & storage Conversion potential subject to the necessary permissions being obtained Garden area Parking spaces Starting Bid : £40,000 The Property A traditional two storey brick built farm building with pantile roof. Currently used as garaging, workshop and storage space the building is ideal for conversion to a two/three bedroom dwelling subject to the necessary planning permission being obtained. The building has a garden area and two parking spaces. An excellent conversion opportunity. APPROXIMATE DIMENSIONS OF PLOT 32 meters by 5.5 meters including garden LOCATION The barn is situated just to the north of the popular Trent side village of Amcotts which is ideally located for access to the motorway network and Scunthorpe. MODERN METHOD OF AUCTION This property is for sale by the Modern Method of Auction which is not to be confused with Traditional auction. The Modern Method of Auction is a flexible buyer friendly method of purchase. We do not require the purchaser to exchange contracts immediately, but grant 28 days to achieve exchange of contracts from the date the buyer's solicitor is in receipt of the draft contracts and a further 28 days thereafter to complete. Allowing the additional time to exchange on the property means interested parties can proceed with traditional residential finance. Upon close of a successful auction or if the vendor accepts an offer during the auction, the buyer will be required to put down a non-refundable Reservation Fee of 5% subject to a minimum of £5,000 plus VAT which secures the transaction and takes the property off the market. -

St Marks Community Group Amcotts
ST MARKS COMMUNITY GROUP AMCOTTS "Amcotts Christmas Lights" on Saturday 10th December 2016 to create a little magic for the children/grandchildren of Amcotts. CHRISTMAS TREES ( If you have booked one of the 25 Christmas Trees for your child / grandchild they should arrive on Saturday December 3rd 2016) PROGRAMME Committee members will help set up stalls during morning of the 10th Dec. 4-30 - 7-00 The New Amcotts Christmas Stalls will be at The Ingleby Arms, Church Street, St Marks Church, The Jubilee Garden, Middle Lane, Chapel Street, Pasture Lane and Dark Lane. Please walk round the village and try to see and support all the stalls. CAKE STALLS in Chapel Street, TEDDY TOMBOLA in Church Street, WINE OR WATER at the Old Post Office, HOT DOGS/BEEF BURGERS at the Ingleby Arms, GINGER BREAD at Old School House, MYSTERIES in Dark Lane plus.... 5-00 - 6-00 School Choirs/Carols in the Jubilee Garden / Light up a Life Tree HEALTH AND SAFETY 1. CAR PARKING will be at Hook Farm and The Ingleby Arms but we hope most people will be walking. 2. TEA and COFFEE will be served in the Church and provide a shelter for those needing to sit down and keep warm. 3. FIRST AID - St John's Ambulance staff will be at the Old Post Office. 4. LOST CHILDREN should be taken to the Old Post Office where Police Community Support Officers will also be available. PROGRAMME contd. 6-00 - 6-45 The Lions Sleigh with Father Christmas aboard will arrive at the Ingleby Arms at approx 6-00 to light their trees.