Bioavailability and Sources of Nutrients and the Linkages to Nuisance Drift Algae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Addison Bay, 64 Advanced Sails, 351
FL07index.qxp 12/7/2007 2:31 PM Page 545 Index A Big Marco Pass, 87 Big Marco River, 64, 84-86 Addison Bay, 64 Big McPherson Bayou, 419, 427 Advanced Sails, 351 Big Sarasota Pass, 265-66, 262 Alafia River, 377-80, 389-90 Bimini Basin, 137, 153-54 Allen Creek, 395-96, 400 Bird Island (off Alafia River), 378-79 Alligator Creek (Punta Gorda), 209-10, Bird Key Yacht Club, 274-75 217 Bishop Harbor, 368 Alligator Point Yacht Basin, 536, 542 Blackburn Bay, 254, 260 American Marina, 494 Blackburn Point Marina, 254 Anclote Harbors Marina, 476, 483 Bleu Provence Restaurant, 78 Anclote Isles Marina, 476-77, 483 Blind Pass Inlet, 420 Anclote Key, 467-69, 471 Blind Pass Marina, 420, 428 Anclote River, 472-84 Boca Bistro Harbor Lights, 192 Anclote Village Marina, 473-74 Boca Ciega Bay, 409-28 Anna Maria Island, 287 Boca Ciega Yacht Club, 412, 423 Anna Maria Sound, 286-88 Boca Grande, 179-90 Apollo Beach, 370-72, 376-77 Boca Grande Bakery, 181 Aripeka, 495-96 Boca Grande Bayou, 188-89, 200 Atsena Otie Key, 514 Boca Grande Lighthouse, 184-85 Boca Grande Lighthouse Museum, 179 Boca Grande Marina, 185-87, 200 B Boca Grande Outfitters, 181 Boca Grande Pass, 178-79, 199-200 Bahia Beach, 369-70, 374-75 Bokeelia Island, 170-71, 197 Barnacle Phil’s Restaurant, 167-68, 196 Bowlees Creek, 278, 297 Barron River, 44-47, 54-55 Boyd Hill Nature Trail, 346 Bay Pines Marina, 430, 440 Braden River, 326 Bayou Grande, 359-60, 365 Bradenton, 317-21, 329-30 Best Western Yacht Harbor Inn, 451 Bradenton Beach Marina, 284, 300 Big Bayou, 345, 362-63 Bradenton Yacht Club, 315-16, -

Compilation of Anchorages in SW Florida
Compilation of Anchorages in SW Florida Document is a compilation of information found from cruisers net and Florida Sea grant publications. Revised 1-2015 CAPE SABLE TO OTTER LIDO KEY ANCHORAGES (Armands circle) CONTENTS 1. Cape Sable Anchorages Lat/Lon: near 25 09.569 North/081 08.623 West...........................................................4 2. Little Shark River Outer Anchorage Lat/Lon: near 25 19.677 North/081 08.801 West..........................................4 3. Little Shark River Southern Fork Anchorage Lat/Lon: near 25 19.736 North/081 07.132 West ............................5 4. Little Shark River Upper Anchorage Lat/Lon: near 25 20.268 North/081 06.983 ..................................................5 5. New Turkey Key Anchorage Lat/Lon: near 25 38.984 North/081 16.759 West .....................................................6 6. Lumber Key Anchorage Lat/Lon: near 25 45.627 North/081 22.835 West ............................................................7 7. Jack Daniels Key Anchorage Lat/Lon: near 25 47.882 North/081 25.931 West....................................................7 8. Kingston Key Anchorage Lat/Lon: near 25 48.005 North/081 27.011 West ..........................................................8 10. Russell Pass Southern Anchorage Lat/Lon: near 25 49.917 North/081 26.516 West.........................................9 11. Russell Pass Middle Anchorage Lat/Lon: near 25 50.303 North/081 26.317 West .......................................... 10 12. Russell Pass Northern Anchorage Lat/Lon: near 25 50.542 North/081 26.019 West....................................... 11 13. Picnic Key Anchorage Lat/Lon: near 25 49.278 North/081 29.116 West.......................................................... 12 14. Caxambas Pass Anchorage Lat/Lon: near 25 54.129 North/081 39.953 West ................................................ 13 15. Coon Key Pass Anchorage Lat/Lon: near 25 54.115 North/081 38.433.......................................................... -
Po ²· I* [T I9
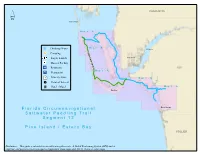
´ CHARLOTTE Boca Grande MM aa pp 11 -- AA op MM aa pp 11 -- BB Drinking Water Ft. Myers a l t[ t Camping e r n Cape Coral Kayak Launch a t e r Shower Facility o u t I* Restroom e LEE MM aa pp 22 -- AA I9 Restaurant ²· Grocery Store MM aa pp 22 -- BB e! Point of Interest l Hotel / Motel MM aa pp 33 -- AA Sanibel Bonita Springs FF ll oo rr ii dd aa CC ii rr cc uu mm nn aa vv ii gg aa tt ii oo nn aa ll SS aa ll tt ww aa tt ee rr PP aa dd dd ll ii nn gg TT rr aa ii ll SS ee gg mm ee nn tt 11 22 PP ii nn ee II ss ll aa nn dd // EE ss tt ee rr oo BB aa yy COLLIER Disclaimer: This guide is intended as an aid to navigation only. A Gobal Positioning System (GPS) unit is required, and persons are encouraged to supplement these maps with NOAA charts or other maps. Naples Segment 12: Pine Island / Estero BayGASPARILLA SOUND-CHARLOTTE HARBOR AQUATIC PRESERVE Map 1 A 18 6 12 3 Bokeelia Island 6 3 A Jug Creek Cottages 6 e! Jug Creek Point 12 Jug Creek 12 3 6 3 3 ´ 6 Little Bokeelia Island Bokeelia Launch 6 N: 26.6942 I W: -82.1459 12 6 Murdock Point 3 3 Cayo Costa Boat Dock Big Smokehouse Key Mondongo Island 6 N: 26.6857 I W: -82.2455 Big Jim Creek Broken Islands Cayo Costa 3 12 6 State Park 3 3 Darling Key Pineland/Randell Research Center Primo Island N: 26.6593 I W: -82.1529 Useppa Island 3 12 MATLACHA PASS 3 3 6 3 Whoopee Island e! Pineland AQUATIC PRESERVE Cayo Costa 3 12 3 A Part Island l 6 3 12 Pine Island te Cabbage Key rn a Black Key te Bear Key R Coon Key o 3 6 u t e Cove Key Narrows Key 3 6 Wood Key Cat Key 6 6 PINE ISLAND SOUND 3 Little Wood -

Currently the Bureau of Beaches and Coastal Systems
CRITICALLY ERODED BEACHES IN FLORIDA Updated, June 2009 BUREAU OF BEACHES AND COASTAL SYSTEMS DIVISION OF WATER RESOURCE MANAGEMENT DEPARTMENT OF ENVIRONMENTAL PROTECTION STATE OF FLORIDA Foreword This report provides an inventory of Florida's erosion problem areas fronting on the Atlantic Ocean, Straits of Florida, Gulf of Mexico, and the roughly seventy coastal barrier tidal inlets. The erosion problem areas are classified as either critical or noncritical and county maps and tables are provided to depict the areas designated critically and noncritically eroded. This report is periodically updated to include additions and deletions. A county index is provided on page 13, which includes the date of the last revision. All information is provided for planning purposes only and the user is cautioned to obtain the most recent erosion areas listing available. This report is also available on the following web site: http://www.dep.state.fl.us/beaches/uublications/tech-rut.htm APPROVED BY Michael R. Barnett, P.E., Bureau Chief Bureau of Beaches and Coastal Systems June, 2009 Introduction In 1986, pursuant to Sections 161.101 and 161.161, Florida Statutes, the Department of Natural Resources, Division of Beaches and Shores (now the Department of Environmental Protection, Bureau of Beaches and Coastal Systems) was charged with the responsibility to identify those beaches of the state which are critically eroding and to develop and maintain a comprehensive long-term management plan for their restoration. In 1989, a first list of erosion areas was developed based upon an abbreviated definition of critical erosion. That list included 217.6 miles of critical erosion and another 114.8 miles of noncritical erosion statewide. -
Southwest Coast Red Tide Status Report June 4, 2021
Red Tide Status - Florida Southwest Coast June 04, 2021 Present Status: The red tide organism, Karenia brevis, persists in Southwest Florida. K. brevis was observed at background and low concentrations in two samples collected from Pinellas County, very low to medium concentrations in seven samples collected from Hillsborough County, very low to medium concentrations in 18 samples collected from Manatee County, background concentrations in one sample collected from Sarasota County, background to low concentrations in 15 samples collected from and offshore of Lee County, and background to medium concentrations in 10 samples collected from and offshore of Collier County. Fish kills suspected to be related to red tide were reported over the past week in Pinellas, Manatee, Lee, and Collier counties. For more details, please visit: https://myfwc.com/research/saltwater/health/fish-kills- hotline/. Respiratory irritation was reported over the past week in Pinellas County (6/1 at Pass-a-Grille) and Collier County. For current information, please visit: https://visitbeaches.org/. Forecasts by the USF-FWC Collaboration for Prediction of Red Tides for Pinellas to northern Monroe counties predict northern movement of surface waters and minimal transport of subsurface waters over the next four days. Date Alongshore County Offshore Site Location Collector Collected Inshore Pinellas - 06/01 not present - Clearwater Beach Pier 60 FWRI Grand Bellagio Condo Dock - 06/01 not present - FWRI (Old Tampa Bay) Bravo Drive; S of (Allens - 06/02 not present - -

March 10 2021
Wednesday Update March 10, 2021 Welcome to the bi-weekly Wednesday Update! We'll email the next issue on March 24. By highlighting SCCF's mission to protect and care for Southwest Florida's coastal ecosystems, our updates connect you to nature. Thanks to Mike Puma for this photo of a tri- colored heron (Egretta tricolor) taken on Sanibel. DO YOU HAVE WILDLIFE PHOTOS TO SHARE? Please send your photos to [email protected] to be featured in an upcoming issue. SCCF Partners with Conservancy to Hire Water Analyst To further a commitment to regional water quality and Everglades restoration through a unified front, SCCF and the Naples-based Conservancy of Southwest Florida have partnered to hire a hydrological modeler. “We better fulfill our west coast mission by pooling our resources and streamlining our efforts,” said SCCF CEO Ryan Orgera, Ph.D. “Doing so, we were able to hire a highly qualified data analyst who will move us more efficiently towards water quality solutions.” On March 16, Paul Julian, Ph.D., will begin working as a hydrological modeler for SCCF and the Conservancy of Southwest Florida. The goal of this partnership is to address an important need for modeling expertise and data analysis in Southwest Florida. Work products will be shared between the two non-profits, which have led conservation efforts in Lee and Collier counties for more than five decades. For the past ten years, Julian worked as the Everglades Technical Lead for the Florida Department of Environment Protection (FDEP). In that role, he gained deep understanding of the dynamic Greater Everglades Ecosystem by performing water quality compliance calculations, supporting federal and state restoration planning efforts, developing water quality nutrient models, and mining and analysis of environmental data. -

Long Term Success and Future Approach of the Captiva and Sanibel Islands Beach Renourishment Program
2017 National Conference on Beach Preservation Technology February 8-10, 2017; Stuart, Florida Long Term Success and Future Approach of the Captiva and Sanibel Islands Beach Renourishment Program Thomas P. Pierro, PE, D.CE, Director, CB&I Michelle Pfeiffer, P.E., Senior Project Engineer, CB&I Stephen Keehn, P.E., Senior Coastal Engineer, CB&I Kathleen Rooker, Adminstrator, CEPD, Captiva, FL Acknowledgments CEPD Board Members, Alison Hagerup, Tom Campbell, Bill Stronge A World of Solutions 2016 Annual Conference Fireside Chat Series . Hurricane Hermine . Windshield inspection 9/2/2016 . Beach buffered storm A World of Solutions 1 Captiva Island Erosion Prevention District . The District was established as a beach and shore preservation district June 19, 1959. “Our sole purpose and dedication is to Captiva beach and shore preservation.” – Kathy Rooker, Captiva Island Erosion Prevention District (2017) . Lee County's Beach Management Plan traces its roots to Captiva Island. “Captiva Island was the birthplace of beach nourishment in Lee County.” – Steve Boutelle, Lee County Division of Natural Resources (2014) A World of Solutions 2 Captiva Beach Culture . Captiva Island property owners overwhelming support beach projects. “Its expected and accepted.” – Longtime property owner regarding the beach nourishment projects A World of Solutions 3 Captiva Island . Lee County . Barrier island system . Connected waterways Captiva Pass North Captiva Island Redfish Pass Pine Island Sound Captiva Island Blind Pass Gulf of Mexico Sanibel Island A World of Solutions 4 Project Location Map . Over 50 years of nourishment projects . Limited fill placements in 1961 and 1981; 134 groins . First island-wide nourishment in 1988/89 . Renourished in 1996 and 2005/06 . -

Charlotte Harbor National Estuary Program Committing to Our Future a Public-Private Partnership to Protect the Charlotte Harbor Estuarine and Watershed System
Charlotte Harbor National Estuary Program Committing To Our Future A Public-Private Partnership to Protect the Charlotte Harbor Estuarine and Watershed System Comprehensive Conservation and Management Plan (CCMP) 2008 Update SUMMARY This summary of the Charlotte Harbor National Estuary Program Comprehensive Conservation and Management Plan (CCMP) was created with information from the CCMP approved in 2001 and 2008 and various sources provided by the U.S. Environmental Protection Agency and the Southwest Florida Regional Planning Council. The CCMP 2008 Update and this document, the CCMP Summary 2008 Update, may be obtained as PDF files from the Program website at www. CHNEP.org. Printed copies are available from the Program Office and can be requested through the website. Charlotte Harbor National Estuary Program 1926 Victoria Avenue, Fort Myers, FL 33901-3414 239/338-2556 | Toll-free 866/835-5785 | Fax 239/338-2560 When an estuary’s www.CHNEP.org components DIRECTOR: Dr. Lisa Beever | [email protected] | ext. 235 SENIOR SCIENTIST: Catherine Corbett | [email protected] | ext. 241 are in balance, DEPUTY DIRECTOR: Liz Donley | [email protected] | ext. 234 COMMUNICATIONS MANAGER: all life flourishes. Maran Hilgendorf | [email protected] | ext. 240 The CCMP Summary was written by Maran Hilgendorf and originally designed by The Set Up, Inc. Photographs were provided by Litaker Photography, Pat and Brady Pfeil, The Ledger, Boca Beacon, Mote Marine Laboratory, Lee County Convention and Visitors Bureau, Charlotte County Convention and Visitors Bureau, Polk County Convention and Visitors Bureau, Sarasota County Convention and Visitors Bureau and CHNEP staff. © Revised January 2008. September 2002. Printed on recycled paper. -

Appendix I: Critical Erosion Report 2018 State Hazard Mitigation Plan ______
Appendix I: Critical Erosion Report 2018 State Hazard Mitigation Plan _______________________________________________________________________________________ APPENDIX I: Critical Erosion Report _______________________________________________________________________________________ Florida Division of Emergency Management Critically Eroded Beaches In Florida Division of Water Resource Management Florida Department of Environmental Protection August 2016 2600 Blair Stone Rd., MS 3590 Tallahassee, FL 32399-3000 www.dep.state.fl.us Foreword This report provides an inventory of Florida’s erosion problem areas fronting on the Atlantic Ocean, Straits of Florida, Gulf of Mexico, and the roughly sixty-six coastal barrier tidal inlets. The erosion problem areas are classified as either critical or non-critical and county maps and tables are provided to depict the areas designated critically and non-critically eroded. Many areas have significant historic or contemporary erosion conditions, yet the erosion processes do not currently threaten public or private interests. These areas are therefore designated as non-critically eroded areas and require close monitoring in case conditions become critical. This report, originating in 1989, is periodically updated to include additions and deletions. All information is provided for planning purposes only and the user is cautioned to obtain the most recent erosion areas listing available in the updated critical erosion report of 2016 on pages 4 through 20 or refer to the specific county of interest listed -

Simulation of Tidal-Flow, Circulation, and Flushing of the Charlotte Harbor Estuarine System, Florida
Simulation of Tidal-Flow, Circulation, and Flushing of the Charlotte Harbor Estuarine System, Florida By Carl R. Goodwin U.S. Geological Survey Water-Resources Investigations Report 93-4153 Prepared in cooperation with the FLORIDA DEPARTMENT OF ENVIRONMENTAL PROTECTION Tallahassee, Florida 1996 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Gordon P. Eaton, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Geological Survey. For additional information Copies of this report can be write to: purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Branch of Information Services Suite 3015 Box 25286 227 N. Bronough Street Denver, CO 80225 Tallahassee, FL 32301 CONTENTS Page Abstract ------------------------------------------------------------------ 1 Introduction -------------------------------------------------------------- 2 Purpose and scope ---------------------------------------------------- 2 Previous studies ----------------------------------------------------- 3 Acknowledgments ------------------------------------------------------ 4 Approach ------------------------------------------------------------------ 4 Description of study area ------------------------------------------------- 5 Physical characteristics --------------------------------------------- 6 Hydrologic characteristics ------------------------------------------- 7 Tidal stage ----------------------------------------------------- -

Blind Pass Inlet Management Study 2018 Update
BLIND PASS INLET MANAGEMENT STUDY 2018 UPDATE Prepared For: Board of County Commissioners of Lee County Prepared By: Aptim Environmental & Infrastructure, Inc. August 2018 BLIND PASS INLET MANAGEMENT STUDY 2018 UPDATE EXECUTIVE SUMMARY Blind Pass is a natural tidal inlet located in Lee County on the Gulf Coast of Florida and is bounded by Captiva Island to the north and Sanibel Island to the south. This area of the coast is characterized by a series of barrier islands and tidal passes that are separated from the mainland of Florida by various water bodies. Blind Pass has migrated and closed at various times throughout history, and is presently managed by Lee County with an ongoing dredging program to maintain the inlet in an open condition. The study described in this document provides an update to the 1993 Blind Pass Inlet Management Plan and recommends refinements for future management of Blind Pass. The study was developed as a collaborative effort with Lee County, the City of Sanibel, and the Captiva Erosion Prevention District (CEPD) to develop a mutually agreeable inlet management strategy for the future in a science-based approach. The study also aligns with the objective of balancing the sediment budget between the inlet and adjacent beaches, and assisting the FDEP in adopting an Inlet Management Plan pursuant to the requirements of Section 161.142, Florida Statutes. The scope of this study included literature review, data collection, preparing a sediment budget update, performing an alternatives analysis with advanced numerical modeling, and developing inlet management recommendations. The alternatives analysis utilized the numerical model Delft3D to evaluate the conceptual designs in an individual and combined fashion. -

Lee County Manatee Protection Plan
LEE COUNTY MANATEE PROTECTION PLAN June 17, 2004 Prepared by: Lee County Division of Natural Resources PO Box 398 Ft. Myers, FL 33902 Lee County Visitor and Convention Bureau Approved: June 29, 2004 John E. Albion, Chairman Lee County Board of County Commissioners Approved: August 24, 2004 Kenneth D. Haddad, Executive Director Florida Fish and Wildlife Conservation Commission Concurrence: August 26, 2004 James Slack, Field Supervisor South Florida Ecological Services Office US Fish and Wildlife Service Executive Summary This Manatee Protection Plan is organized to present first a discussion of manatees and an analysis of manatee abundance in Lee County. An evaluation of boating activity , and the identification of evaluation criteria for the development or expansions of boat facilities follow this. Finally, thresholds and policies are presented to outline Boat Facility siting criteria in Lee County. Once finalized and approved by all parties, these thresholds and policies will explain how the plan is to be used and how preferred boat facility projects differ from non-preferred projects. TABLE OF CONTENTS Executive Summary ......................................................................................................ii List of Figures...............................................................................................................iii List of Tables ................................................................................................................iv List of Appendices .......................................................................................................iv