Quantifying the Risk of Rabies in Biting Dogs in Haiti Xiaoyue Ma1*, Jesse D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bite, Rabies, and Quarantine Information
Rabies and Vaccinations Rabies is a disease caused by a virus (Lyssavirus) found in the saliva of infected animals and is transmitted to other warm- blooded animals, including humans by a bite, scratch or through an open cut. The virus infects Always be alert and know your dog. Watch for signs your dog the central nervous system, causing swelling in is uncomfortable or feeling aggressive and be on the the brain and ultimately death. lookout for potentially dangerous situations. To protect the public, California requires Animal Services to quarantine dogs and cats that have: bitten a human causing a break in the skin, were imported, or exposed to potentially rabid animals, for signs of rabies, for a minimum of 10 days. Dogs are required to be vaccinated for rabies at four months of age. Cats can be vaccinated as early as three months. The first rabies vaccine is What happens during a quarantine? If a dog bite occurs effective for one year. After that initial shot, your Your pet will be placed under quarantine (isolation) 1. Move victim to a safe area. pet should be re-vaccinated every three years. for 10 days to be observed for signs of rabies; this 2. Thoroughly wash the wound with soap and warm Rabies Vaccinations are inexpensive and must allows Animal Services to contain possible rabies water. be administered by a licensed veterinarian. outbreaks. Quarantine can be done at your home if 3. Seek advanced medical aid if necessary; call 9-1-1. Check with Hesperia Animal Services or your authorities are satisfied with the containment area, 4. -

Rabies Information for Dog Owners
Rabies Information for Dog Owners Key Facts Disease in dogs: • During initial days of illness, signs can be nonspecific, such as fever, anxiety and consumption of foreign items (e.g. blankets) • Progresses to more severe signs, such as: • Behavioral change (e.g. aggression, excitability) • Incoordination, loss of balance, disorientation, weakness • Hypersalivation • Seizures • Death results within 10 days of first signs of illness Rabies in dogs is not treatable. Vaccination is key to prevention: • Rabies vaccines are protective if given before exposure to the rabies virus. • Proof of dog vaccination is mandated by many jurisdictions and required for international travel. • Dogs not current on vaccination that are likely exposed to the rabies virus may be required to be euthanized or undergo a long and expensive quarantine. What is it? Rabies is caused by infection with the rabies virus. In North America, the most common wildlife rabies The virus lives in various species of mammals and species (termed reservoirs) vary regionally and is most commonly spread through bites from one include raccoons, skunks, foxes, coyotes, and animal to another or to a human (i.e. in an infected bats. Each year in the United States over 4,000 animal’s saliva). rabid animals are reported, including several Disease in dogs may begin with vague signs of hundred rabid dogs and cats, other domestic illness, but rapidly progresses to severe neurologic species (e.g., horses, cattle, sheep, goats) and signs (e.g. aggression, incoordination). Typically, thousands of wildlife animals. death occurs within 10 days of the first signs of illness. Where is it? The rabies virus is present in nearly all parts of the world. -

Birmans: What a Unique Breed! PET MEDICAL CENTER
Birmans: What a Unique Breed! Your cat is special! She senses your moods, is curious about your day, and has purred her way into your heart. Chances are that you chose her because you like Birmans (sometimes called “Mitted Cats”) and you expected her to have certain traits that would fit your lifestyle, like: Might "chirrup" or trill her meows to call you when she misses you An affectionate companion and family cat Good with children and other pets Requires minimal grooming Excellent companion However, no cat is perfect! You may have also noticed these characteristics: Needs regular exercise and diet regulation to avoid weight gain Needs the company of other pets or people and does not do well in isolation Exhibits signs of separation anxiety if left alone too much Is it all worth it? Of course! She's full of personality, and you love her for it! She is gentle, friendly and loves to be involved in your daily activities. The origin of the Birman remains a mystery with many unverified tales of their beginnings. Born all white, Birmans do not develop their full color until maturity. They keep their unique white “mittens” on all four paws. Birmans are very social and happy to be involved in your daily activities. They are less apt to climb and jump onto high places, preferring to hang out at ground level or on the couch. Birmans have a playful side and will play fetch or chase when engaged, but prefer to lounge with their family. PET MEDICAL CENTER 501 E. FM 2410 ● Harker Heights, Texas 76548 (254) 690-6769 www.pet-medcenter.com cat's dietary habits is key. -
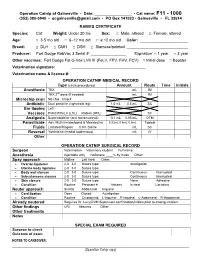
Operation Catnip Medical Records
Operation Catnip of Gainesville • Date: _________________ • Cat name: F11 - 1000 (352) 380-0940 • [email protected] • PO Box 141023 • Gainesville • FL 32614 RABIES CERTIFICATE Species: Cat Weight: Under 20 lbs Sex: □ Male, altered □ Female, altered Age: □ 3-5 mo old □ 6–12 mo old □ ≥ 12 mo old Color: ______________________ Breed: □ DLH □ DMH □ DSH □ Siamese/pointed ____________________________ Producer: Fort Dodge RabVac 3 Serial #: ________________ Expiration: □ 1 year □ 3 year Other vaccines: Fort Dodge Fel-O-Vax LVK III (FeLV, FPV, FHV, FCV) □ Initial dose □ Booster Veterinarian signature: __________________________________ Veterinarian name & license #: __________________________________ OPERATION CATNIP MEDICAL RECORD Type (circle procedures) Amount Route Time Initials Anesthesia TKX mL IM TKX 2nd dose (if needed) mL IM Microchip scan No chip Chip # Antibiotic Dual penicillin (right front leg) 1.0 mL 0.5 mL SC Ear tipping Left Vaccines FVRCP/FeLV (LHL) Rabies (RHL) SC Analgesia Buprenorphine (oral transmucosal) 0.1 mL 0.05 mL OTM Parasiticide Adv. Multi (Imidacloprid & Moxidectin) 0.23mL 0.4mL 0.8mL Topical Fluids Lactated Ringers 0.9% Saline mL SC Reversal Yohimbine (medial saphenous) mL IV Other OPERATION CATNIP SURGICAL RECORD Surgeon Veterinarian Veterinary student Full name: Anesthesia Injectable only Isoflurane ____% by mask Other: Spay approach Midline Left flank Other: Ovarian ligatures 2-0 3-0 Suture type: Autoligation Uterine body ligatures 2-0 3-0 Suture type: Body wall closure 2-0 3-0 Suture type: Continuous Interrupted Subcutaneous closure 2-0 3-0 Suture type: Continuous Interrupted Skin closure 2-0 3-0 Suture type: None Adhesive Condition Routine Pregnant #_______ fetuses In heat Lactating Neuter approach Scrotal Abdominal Inguinal Cord ligation Open Closed Autoligation Condition Routine Cryptorchid: L-Inguinal R-Inguinal L-Abdominal R-Abdominal Already neutered Requires Dr. -

Volunteer Application: Foster Program A. Personal Profile Name
OFFICE USE ONLY Date received: _______________ Orientation date: _____________ Confirmed for FO: □ Yes □ No Volunteer Application: Foster Program A. Personal Profile Name: Address: Postal Code Phone: Home ( ) Work ( ) Cell ( ) E-mail: Do you have access to a vehicle? Yes □ No□ 1. Housing status (check all that apply): □ Own □ Rent □ House □ Apartment □ Townhouse/duplex □ Balcony □ Elevator □ Fenced Yard; Height of fence______ft 2. Are there children living in or frequently visiting your home? □ Yes □ No a. If yes, please list their ages: ________________________________________________ b. Are they comfortable around animals? □ Yes □ No i. If yes, what are they comfortable around? □ Cats □ Dogs □ Small Animals 3. Are there any other members in your household (over the age of 18) who will be assisting you as a foster volunteer? □ Yes □ No a. If yes, please list their name(s) and your relation(s): ______________________________________________________________________________ __________________________________________________________________ b. Are they willing to attend a foster volunteer orientation too? □ Yes □ No B. Becoming a Foster Volunteer 1. Why do you want to become a foster volunteer? 2. Please list any other foster or rescue programs that you volunteer your time with (including feral cats): 3. Please provide a brief description of your experience with very young, ill, injured, or under socialized animals: 5. Please indicate the amount of time per day that you have to dedicate to your foster animal(s): 6. How many hours will your foster animal(s) be alone on a regular basis? 7. We ask foster volunteers to make a commitment of one year to the foster program. Is there anything in the next year that will prevent you from maintaining this commitment (for example: traveling down south for the winter)? 8. -

What Is Rabies and How Do People Get It? Why Should I Learn About Bats
What is rabies and how do people get it? Rabies is an infectious viral disease that affects the nervous system of humans and other mammals. People get rabies from the bite of an animal with rabies. Raccoon, skunks, foxes, coyotes, and bats can have rabies and can transmit it to people. If untreated, rabies is a fatal disease. The goal of public health is to prevent human exposure to rabies by education and to prevent the disease by anti-rabies treatment if exposure occurs. Tens of thousands of people are successfully treated each year after being bitten by an animal that may have rabies. A few people die of rabies each year in the United States, usually because they do not recognize the risk of rabies from the bite of a wild animal and do not seek medical advice. Why Should I Learn About Bats and Rabies? Most of the recent human rabies cases in the United States have been caused by rabies exposure from bats. Awareness of the facts about bats and rabies can help people protect themselves, their families, and their pets. When people think about bats, they often imagine things that are not true. Bats are not blind. They are neither rodents nor birds. They will not suck your blood -- and most do not have rabies. Bats play key roles in ecosystems around the globe, from rain forests to deserts, especially by eating insects, including agricultural pests. The best protection we can offer these unique mammals is to learn more about their habits and recognize the value of living safely with them. -

The Rabid Cat of Lane County Oregon Epidemiologists’ Meeting 2018
Ducky… the Rabid Cat of Lane County Oregon Epidemiologists’ Meeting 2018 October 9 2018 Sarah Puls, REHS, MS Heather Young, RN, BS Rabies History in Lane County . Rabies is endemic in Oregon . Rare in dogs and cats . No cats had tested positive for rabies . Dog tested positive in the 1980’s Background: Rabies Virus .Family Rhabdoviridae, genus Lyssavirus .Clinically: acute viral zoonotic disease .Classical rabies virus only species in U.S. .Causes 99.9% infections worldwide .55,000 people still die yearly from rabies May 8, 2017 . Lane County EH received a call from a local veterinary clinic reporting a cat bit a veterinary technician . Veterinarian thought this cat was acting abnormal . Local animal control officer thought animal was acting abnormal . The situation surrounding the bite was suspicious. Owner opted for euthanization and head was shipped to OSPHL Rabies Transmission .Spread through saliva from infected animal .Can also be spread through brain tissue .Travels via nerves to spinal cord and brain May 10, 2017 . Dr. DeBess reported positive rabies test for cat . Follow up with the veterinary clinic and victim was immediately initiated . Follow up with the animal owner was immediately initiated . Notification to the LC Communicable Disease . Information entered into Orpheus Also Wednesday May 10, 2017 CD Nurse gets a call from EH . And does some research… Post-Exposure Rabies Prophylaxis Rabies Vaccine RIG U.S. Vaccine Schedule: 4 doses days 0, 3, 7, 14 RIG infused around wound on day 0 Springfield Animal Control Coordination . Consults with local vet clinics . Consults with Public Health . Educating the community on the care and laws regarding pets. -

8 Things You May Not Know About Rabies – but Should for #Worldrabiesday, Two Experts Share Less-Known Facts About Rabies and How to Prevent It by Pamela J
8 things you may not know about rabies – but should For #WorldRabiesDay, two experts share less-known facts about rabies and how to prevent it By Pamela J. Wilson, LVT, MEd, and Rodney E. Rohde, PhD Rabies is a viral zoonosis affecting the central nervous system of warm-blooded animals. Transmission occurs when saliva containing the rabies virus is introduced into an opening in the skin, usually via the bite of a rabid animal. Though rare, transmission could occur through infected saliva contacting mucous membranes or a scratch or other break in the skin. After the rabies virus enters the body, it begins to multiply in the area near the entry site. If the infection is not World Rabies Day stopped at this point, the virus will eventually invade the Monday, September nerve cells in that area. Once the virus is in the nerve 28, is World Rabies tissue, it travels along the nerve to the brain, where it Day, and this year's continues to multiply. The virus may then spread along theme is "End nerves from the brain to the salivary glands or other parts Rabies Together." of the body. On Twitter, the hashtags are Rabies remains a dreaded disease because it is almost #WorldRabiesDay always fatal, plus it is accompanied by distressing clinical and #EndRabies. signs and symptoms; however, if rabies postexposure You can learn more on the website of the Global prophylaxis (PEP) is administered before the virus enters Alliance for Rabies Control. the nervous system, it can be prevented. Despite its severity, there is still much the public and even health professionals may not know about rabies. -

Pet Information Vaccine Information Administered By: Rabies Certificate
Noah Keighley 1525 Aschinger Blvd Rabies Certificate Columbus, OH 43212 Home Phone: (614) 202-5804 Certificate Date Cell Phone: (614) 202-5804 Banfield Pet Hospital [email protected] 1541 W Lane Ave, Friday, May 29, 2020 11:11 AM Upper Arlington, OH 43221-3221 (614) 487-0195 Pet Information Rabies Tags Name: Dakota Keighley Species: Canine Banfield Breed: Australian Cattle Dog (Mix) National Pet ID# Gender: Female 6820990 Color: Tan/white County & Tag # Age: 17 weeks Franklin Weight: 25.60 lbs A428760 Microchip ID: Manufacturer: Microchip ID: Manufacturer: Vaccine Information Vaccine Name : Rabies (Type: Killed) Producer: Zoetis Defensor 3 Administered: 5/29/2020 Route: Subcutaneous Site: Right Rear Due Date: 5/29/2021 Lot/Serial #: 390138A Lot/Serial Expires: 5/18/2021 I hereby certify that I have vaccinated this pet in accordance with all state and Federal laws and regulations on this date. Administered by: Licensed Veterinarian: Dr. Molly Anne Watson DVM License Number: VET11004 Any rabies certificate for this pet printed prior to the date of this certificate is null and void ReportID: RabiesCert.en.x Hospital #5167 Page 1 of 1 Time Printed: 11:11 AM Date Printed: 5/29/2020 Noah Keighley 1525 Aschinger Blvd Columbus, OH 43212 Home Phone: (614) 202-5804 Outpatient Therapy Cell Phone: (614) 202-5804 Recommendations [email protected] Banfield Pet Hospital 1541 W Lane Ave Friday, May 29, 2020 11:11 AM Upper Arlington, OH 43221-3221 (614) 487-0195 Patient: Dakota Visit Date: 5/29/2020 Presenting Complaint: Preventive Care -- Vaccination/Deworming Topic: ASCARIDS, ROUNDWORMS Ascarids, or roundworms, are common intestinal parasites found in dogs and cats. -

Rabies Bites! the 10-Day Quarantine Period Ensures That the Dog Or Cat Remains Available So That It Can Be Observed for Signs of Rabies
Why require a 10-day quarantine period? Rabies Bites! The 10-day quarantine period ensures that the dog or cat remains available so that it can be observed for signs of rabies. If the animal remains well during the 10 days, this indicates it did not have the rabies virus in its saliva at the time of the bite, and therefore the bite victim does not have to receive an expensive and unpleasant series of shots to prevent rabies. This is why it is so important that the dog or cat under Wisconsin state law requires that quarantine be strictly confined at all times to ensure that it cannot run away or be injured. any dog or cat that bites a person be The 10-day confinement and observation period quarantined for ten days so that it for dogs and cats that bite humans has stood the can be observed for signs of rabies. test of time as a way to prevent human rabies. This quarantine period avoids the need to destroy the biting This brochure explains what such a dog or cat in order to test its brain for the rabies virus. quarantine involves and gives the reasons why these measures must be What happens if I don’t comply? taken. Note that the information in this State law provides for a fine of $100 to $1,000 or brochure applies only to dogs and cats 60 days imprisonment or both for failure to comply that have bitten a person, and that the with a quarantine order. requirements of the quarantine vary depending on whether the animal is Where can I find more information? current on its rabies immunizations. -

Animal Bites and Rabies Risk Manual
ANIMAL BITES RABIES RISK a guide for health professionals For Consultation on Animal Bites and Rabies Risk in Humans Minnesota Department of Health Zoonotic Diseases Unit 625 North Robert Street St. Paul, MN 55155 Telephone: 651-201-5414 or toll free: 1-877-676-5414 • 24/7 telephone consultation on potential rabies exposure is available to healthcare providers, veterinarians, public health professionals, and law enforcement. • Rabies consultations are available to the public Monday through Friday, 8:30 a.m. to 4:30 p.m. For Consultation on Rabies Exposure of Animals Minnesota Board of Animal Health 625 North Robert Street St. Paul, MN 55155 Telephone: 651-201-6808 For Specimen Submission Specimens from suspect rabies animals should be delivered to: Business hours (Monday - Friday, 8:00 a.m. to 4:30 p.m.) Minnesota Veterinary Diagnostic Laboratory University of Minnesota-St. Paul Campus 1333 Gortner Avenue St. Paul, MN 55108 Phone: 612-625-8787 - 2 - ANIMAL BITES AND RABIES RISK: A GUIDE FOR HEALTH PROFESSIONALS Table of Contents I. INTRODUCTION ............................................................................................................ 5 II. MANAGEMENT OF ANIMAL BITES TO HUMANS ......................................................... 5 Consultations on animal bites and rabies risk ........................................................................5 Evaluation of the patient following an animal bite ...............................................................5 Assessment of the need for rabies post-exposure prophylaxis -

Dirofilariosis in the Americas: a More Virulent Dirofilaria Immitis? Filipe Dantas-Torres1,2* and Domenico Otranto2
Dantas-Torres and Otranto Parasites & Vectors 2013, 6:288 http://www.parasitesandvectors.com/content/6/1/288 REVIEW Open Access Dirofilariosis in the Americas: a more virulent Dirofilaria immitis? Filipe Dantas-Torres1,2* and Domenico Otranto2 Abstract Dirofilarioses are widespread diseases caused by filarioid nematodes (superfamily Filarioidea) of the genus Dirofilaria, which are transmitted by a plethora of mosquito species. The principal agent of canine dirofilariosis in the Americas is Dirofilaria immitis, which may also occasionally infest humans, resulting in pulmonary nodules that may be confounded with malignant lung tumours. Because human cases of dirofilariosis by D. immitis are relatively frequent in the Americas and rare in Europe and other eastern countries, where Dirofilaria repens is the main causative agent, the existence of a more virulent strain of D. immitis in the Americas has been speculated. Recently, a case of human ocular infestation by Dirofilaria sp. was diagnosed in Pará State, northern Brazil, where canine heartworm dirofilariosis is endemic. The nematode was shown to be morphologically and phylogenetically related to D. immitis but it was genetically distinct from reference sequences, including those of D. immitis infesting dogs in the same geographical area. This finding raised questions regarding the aetiology of human dirofilariosis in the Americas, since information on the genetic makeup of filarioids infesting dogs and humans is meagre. Further studies would be needed to better characterize filarioids infesting dogs, wild animals, and humans in the Americas and to assess the existence of a more virulent D. immitis strain in this continent. Finally, the competence of different culicid species/strains from Europe and the Americas as vectors of Dirofilaria species should be investigated.