A Study on the Geochemistry of Fluoride in Groundwater for the Delineation of Fluoride Zones
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LHA Recuritment Visakhapatnam Centre Screening Test Adhrapradesh Candidates at Mudasarlova Park Main Gate,Visakhapatnam.Contact No
LHA Recuritment Visakhapatnam centre Screening test Adhrapradesh Candidates at Mudasarlova Park main gate,Visakhapatnam.Contact No. 0891-2733140 Date No. Of Candidates S. Nos. 12/22/2014 1300 0001-1300 12/23/2014 1300 1301-2600 12/24/2014 1299 2601-3899 12/26/2014 1300 3900-5199 12/27/2014 1200 5200-6399 12/28/2014 1200 6400-7599 12/29/2014 1200 7600-8799 12/30/2014 1177 8800-9977 Total 9977 FROM CANDIDATES / EMPLOYMENT OFFICES GUNTUR REGISTRATION NO. CASTE GENDER CANDIDATE NAME FATHER/ S. No. Roll Nos ADDRESS D.O.B HUSBAND NAME PRIORITY & P.H V.VENKATA MUNEESWARA SUREPALLI P.O MALE RAO 1 1 S/O ERESWARA RAO BHATTIPROLU BC-B MANDALAM, GUNTUR 14.01.1985 SHAIK BAHSA D.NO.1-8-48 MALE 2 2 S/O HUSSIAN SANTHA BAZAR BC-B CHILAKURI PETA ,GUNTUR 8/18/1985 K.NAGARAJU D.NO.7-2-12/1 MALE 3 3 S/O VENKATESWARULU GANGANAMMAPETA BC-A TENALI. 4/21/1985 SHAIK AKBAR BASHA D.NO.15-5-1/5 MALE 4 4 S/O MAHABOOB SUBHANI PANASATHOTA BC-E NARASARAO PETA 8/30/1984 S.VENUGOPAL H.NO.2-34 MALE 5 5 S/O S.UMAMAHESWARA RAO PETERU P.O BC-B REPALLI MANDALAM 7/20/1984 B.N.SAIDULU PULIPADU MALE 6 6 S/O PUNNAIAH GURAJALA MANDLAM ,GUNTUR BC-A 6/11/1985 G.RAMESH BABU BHOGASWARA PET MALE 7 7 S/O SIVANJANEYULU BATTIPROLU MANDLAM, GUNTUR BC-A 8/15/1984 K.NAGARAJENDRA KUMAR PAMIDIMARRU POST MALE 8 8 S/O. -

2017-18 Srikakulam District
DISTRICT HAND BOOK OF STATISTICS ———2018—201820182018 Lakshminarsupeta SRIKAKULAM DISTRICTDISTRICTDISTRICT HAND BOOK OF STATISTICS -2017-18 SRIKAKULAM DISTRICT COMPILED & PUBLISHED BY CHIEF PLANNING OFFICER SRIKAKULAM SRI K.D HANUNJAYA REDDY , I.A.S., Collector & District Magistrate, Srikakulam. PREFACE th The “HAND BOOK OF STATISTICS” for the year 2018 is the 35 edition in this series. It contains valuable Statistical Data relating to different Sectors and Departments in Srikakulam District. Basic data is a prime requisite in building up strategic plans with time bound targets. I hope this publication will be very useful to all General Public, Planners, Research Scholars, Administrators, Bankers and Other Organizations. I am very much thankful to all the District Officers for extending their co-operation in supplying the data relating to their sectors to bring out this publication as a ready reckoner. I appreciate the efforts made by Sri M.Mohana Rao, Chief Planning Officer , Srikakulam and his staff members for the strenuous efforts in compiling and bringing out the “HAND BOOK OF STATISTICS” for the year 2018. Any constructive suggestion for improvement of this publication and coverage of Statistical Data would be appreciated. Date: Place: Srikakulam. District Collector, Srikakulam. OFFICERS AND STAFF ASSOCIATED WITH THE PUBLICATION 1. SRI M.MOHANA RAO CHIEF PLANNING OFFICER 2. SMT V.S.S.L.PRASANNA DEPUTY DIRECTOR 3. SRI A. SALEM RAJU ASSISTANT DIRECTOR 4. SRI J.TIRUPATHI RAO STATISTICAL OFFCIER DATA COMPILATION: 1. SRI D.VENKATARAMANA DY. STATISTICAL OFFICER DATA PROCESSING & COMPUTERISATION : 1. SRI D.VENKATARAMANA DY. STATISTICAL OFFICER 2. SRI P.YOGESWARA RAO COMPUTER OPERATOR CONTENTS TABLE CONTENTS PAGE NO NO. -

Hand Book of Statistics Srikakulam District 2013
HAND BOOK OF STATISTICS SRIKAKULAM DISTRICT 2013 COMPILED & PUBLISHED BY CHIEF PLANNING OFFICER SRIKAKULAM DR.P.Laxminarasimham, I.A.S., Collector & District Magistrate, Srikakulam. Photograph of the District Collector PREFACE th The “HAND BOOK OF STATISTICS” for the year 2013 is 30 in its series. It contains valuable Statistical Data relating to different Sectors and Departments in Srikakulam District. Basic data is a prime requisite in building up strategic plans with time bound targets. I hope this publication will be very useful to all General Public, Planners, Research Scholars, Administrators, Bankers and Other Organizations. I am very much thankful to all the District Officers for extending their co-operation in supplying the data relating to their sectors to bring out this publication as a ready reckoner. I appreciate the efforts made by Sri M.Sivarama Naicker, Chief Planning Officer, Srikakulam and his staff members for the strenuous efforts in compiling and bringing out the “HAND BOOK OF STATISTICS” for the year 2013. Any constructive suggestion for improvement of this publication and coverage of Statistical Data would be appreciated. Date: -02-2015, Place: Srikakulam. District Collector, Srikakulam. CONTENTS TABLE CONTENTS PAGE NO NO. GENERAL A SALIENT FEATURES OF SRIKAKULAM DISTRICT - PLACES OF TOURIST IMPORTANCE i - xi B COMPARISON OF THE DISTRICT WITH THE STATE 1 - 5 C ADMINISTRATIVE DIVISIONS IN THE DISTRICTS 6 C1 MUNICIPAL INOFMRATION IN THE DISTRICT 7 D PUBLIC REPRESENTATIVES / NON OFFICIALS 8-9 E PROFILE OF ASSEMBLY -

Hand Book of Statistics-2015 Srikakulam District
HAND BOOK OF STATISTICS-2015 SRIKAKULAM DISTRICT COMPILED & PUBLISHED BY CHIEF PLANNING OFFICER SRIKAKULAM DR.P.Laxminarasimham, I.A.S., Collector & District Magistrate, Srikakulam. PREFACE The “HAND BOOK OF STATISTICS” for the year 2015 is 32nd in its series. It contains valuable Statistical Data relating to different Sectors and Departments in Srikakulam District. Basic data is a prime requisite in building up strategic plans with time bound targets. I hope this publication will be very useful to all General Public, Planners, Research Scholars, Administrators, Bankers and Other Organizations. I am very much thankful to all the District Officers for extending their co-operation in supplying the data relating to their sectors to bring out this publication as a ready reckoner. I appreciate the efforts made by Sri M.Sivarama Naicker, Chief Planning Officer, Srikakulam and his staff members for the strenuous efforts in compiling and bringing out the “HAND BOOK OF STATISTICS” for the year 2015. Any constructive suggestion for improvement of this publication and coverage of Statistical Data would be appreciated. Date: -11-2016, Place: Srikakulam. District Collector, Srikakulam. OFFICERS AND STAFF ASSOCIATED WITH THE PUBLICATION 1.SRI. M.SIVARAMA NAICKER CHIEF PLANNING OFFICER 2.SRI. CH.VASUDEAVRAO DEPUTY DIRECTOR 3.SMT. VSSL PRASANNA ASSISTANT DIRECTOR 4.SRI. V.MALLESWARA RAO STATISTICAL OFFICER 5.SRI. J.LAKSHMANA RAO STATISTICAL OFFCIER DATA COMPILATION: 1. SRI. D.VENKATARAMANA DY. STATISTICAL OFFICER 2. SRI. D.SASIBHUSHANA RAO DY. STATISTICAL OFFICER DATA PROCESSING & COMPUTERISATION: 1. SRI. D.VENKATARAMANA DY. STATISTICAL OFFICER 2. SRI. D.SASIBHUSHANA RAO DY. STATISTICAL OFFICER 3. SRI. P.YOGESWARA RAO COMPUTER OPERATOR CONTENTS TABLE CONTENTS PAGE NO NO. -

Government of a BIDDING DO Project: APR to PROVIDE RURAL
Government of Andhra Pradesh BIDDING DOCUMENT BID . NCB /IFB No: 27/ APRRP/PRED/SKLM District/ROAD/003,2018 -19, Dt.16-07-2018 Project: APRRP (AIIB) TO PROVIDE RURAL ROAD CONNECTIVITY TO UNCONNECTED HABITATIONS OF 250+ POPULATION. Package No: 3/APRRP/PRED/SKLM/Road/003 consists of 94 road works District : Srikakulam Employer: Panchayat Raj Engineering Department, State: Andhra Pradesh. Country: India NATIONAL COMPETITIVE BIDDING (Two-Envelope on line Bidding Process on (e-Procure.gov.in) (FOR % Basis -CIVIL WORKS Packages) Package No: 3/APRRP/PRED/SKLM/Road/003 consists of 94 road works Consists of : 94 road works in Srikakulam District with an ECV of Rs: 7480.89 Lakhs . NAME OF WORK : 3/APRRP/PRED/SKLM/Road/003 consists of 94 road works in Srikakulam District Estimate Contract Value Rs: 7480.89 Lakhs PERIOD OF SALE OF : 23-07-2018, 11.00 HOURS FROM BIDDING DOCUMENT : To: 23-08-2018 15.00 HOURS TIME AND DATE OF : DATE 02-08-2018 TIME 11.00 HOURS PRE-BID CONFERENCE LAST DATE AND TIME FOR :DATE 23-08-2018 TIME 15:30 HOURS RECEIPT OF BIDS * TIME AND DATE OF OPENING :DATE:28-08-2018 TIME 11.00 HOURS HOURS OF BIDS – Technical Part 1 PLACE OF OPENING OF BIDS : O/o of the Engineer-in-Chief, Panchayat Raj, Vijayawada ............................................ OFFICER INVITING BIDS : Engineer-In-Chief, PR, Vijayawada * Should be 2 days after the deadline for submission of bids to allow submission of original documents like bid security etc . 1The firms that qualify technically shall be notified subsequently for opening of the financial part of their bids. -
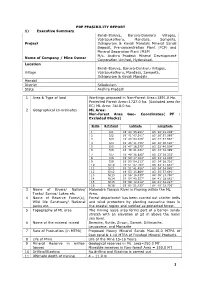
Pre Feasibility Report
PRE FEASIBILITY REPORT 1) Executive Summary Bendi-Baruva, Baruva-Donkuru Villages, Vajrapukothuru, Mandasa, Sompeta, Project Itchapuram & Kavali Mandals Mineral Sands Deposit, Pre-concentration Plant (PCP) and Mineral Separation Plant (MSP) M/s. Andhra Pradesh Mineral Development Name of Company / Mine Owner Corporation Limited, Hyderabad. Location Bendi-Baruva, Baruva-Donkuru Villages, Village Vajrapukothuru, Mandasa, Sompeta, Itchapuram & Kavali Mandals Mandal District Srikakulam State Andhra Pradesh 1 Area & Type of land Workings proposed in Non-Forest Area=1891.0 Ha. Protected Forest Area=1727.0 ha. (Excluded area for EC) ML Area: 3618.0 ha. 2 Geographical co-ordinates ML Area: Non-forest Area Geo- Coordinates( PF : Excluded Blocks) Sl.No B.P.Point Latitude Longitude 1 S/1 180 40’ 35.991” 830 26’ 21.228” 2 S/2 180 41’ 47.541” 830 26’ 57.088” 3 S/3 180 43’ 03.029” 830 27’ 47.562” 4 S/4 180 45’ 11.726” 830 30’ 00.620” 5 S/5 180 47’ 16.570” 830 32’ 40.504” 6 S/6 180 49’ 21.031” 830 33’ 32.399” 7 S/7 180 49’ 36.830” 830 33’ 39.333’ 8 S/8 180 50’ 27.003” 830 33’ 14.865” 9 S/9 180 50’ 54.113” 830 34’ 28.710” 10 S/10 180 51’ 07.703” 830 34’ 41.637” 11 S/11 180 51’ 41.458” 830 34’ 54.045” 12 S/12 180 53’ 35.869” 830 35’ 57.691” 13 N/13 180 56’ 16.539” 840 38’ 13.780” 14 N/14 180 58’ 49.557” 840 41’ 18.861” 15 N/15 180 59’ 12.414” 840 41’ 34.227” 16 N/16 190 04’ 51.435” 840 45’ 32.736” 3 Name of Rivers/ Nallahs/ Mahendra Tanaya River is Flowing within the ML Tanks/ Spring/ Lakes etc Area. -

Mandal Wise Revenue Villages in Srikakulam District Revenue Mandal Sl
Mandal wise Revenue Villages in Srikakulam District Revenue Mandal Sl. Revenue Mandal Village wise Sl. Revenue Village Name No Division Code No 1 Palakonda Bhamini 580070 1 PALAVALASA 2 Palakonda Bhamini 580071 2 MANUMAKONDA 3 Palakonda Bhamini 580072 3 BATTALI 4 Palakonda Bhamini 580073 4 BOMMIKA 5 Palakonda Bhamini 580074 5 PAKKUDIBHADRA 6 Palakonda Bhamini 580075 6 VADDANGI 7 Palakonda Bhamini 580076 7 LOHARIJOLA 8 Palakonda Bhamini 580077 8 GURANDI 9 Palakonda Bhamini 580078 9 NERADI 10 Palakonda Bhamini 580079 10 NULAKAJODU 11 Palakonda Bhamini 580080 11 SINGIDI 12 Palakonda Bhamini 580081 12 PASUKUDI 13 Palakonda Bhamini 580082 13 BURUJOLA 14 Palakonda Bhamini 580083 14 MANIGA 15 Palakonda Bhamini 580084 15 BHAMANI 16 Palakonda Bhamini 580085 16 LIVIRI 17 Palakonda Bhamini 580086 17 DIMMIDIJOLA 18 Palakonda Bhamini 580087 18 SOLIKIRI 19 Palakonda Bhamini 580088 19 GHANASARA 20 Palakonda Bhamini 580089 20 KOSALI 21 Palakonda Bhamini 580090 21 PEDADIMILI 22 Palakonda Bhamini 580091 22 CHINADIMILI 23 Palakonda Hiramandalam 580693 1 KOMANAPALLE 24 Palakonda Hiramandalam 580694 2 GULUMURU 25 Palakonda Hiramandalam 580695 3 JAGANNADHAPURAM 26 Palakonda Hiramandalam 580696 4 KONDARAGOLU 27 Palakonda Hiramandalam 580697 5 PEDDASANKILI 28 Palakonda Hiramandalam 580698 6 DUGGUPURAM 29 Palakonda Hiramandalam 580699 7 BOMMIKA 30 Palakonda Hiramandalam 580700 8 DURBALAPURAM 31 Palakonda Hiramandalam 580701 9 TUNGATAMPARA 32 Palakonda Hiramandalam 580702 10 CHORLANGI 33 Palakonda Hiramandalam 580703 11 M AVALANGI 34 Palakonda Hiramandalam 580704 -

MTS – Candidates Called for Screening Test on 8-8-2021
1 NATIONAL INSTITUTE OF AYURVEDA VACANCY NOTIFICATION NO. 1/2020 SCREENING TEST ON 8-8-2021 (SUNDAY) FROM 9 AM TO 10-30 AM FOR THE POSTS OF MULTI TASKING STAFF (M T S) A Screening Test to shortlist Candidates for Selection to the Post of MTS will be held on SUNDAY 8-8-2021 from 9-00 AM to 10-30 AM in the following Centres. Call Letters have been sent to the Candidates by Speed Post. The List of Candidates Called for Screening Test is given in the following Pages. The List of Applicatons rejected along with the reasons thereof, have also been posted on the Website. Candidates should report for the Screening Test at 8-00 AM at the alloted Centres and should bring the Call Letter and also Proof of Identity like Aadhar Card, Voter ID Card, Driving Licence etc. without which they will not be permitted to appear in the Test. It may be noted that conduct of the Screening Tests will be subject to any Order/Guideline on Covid-19 that may be imposed by State and/or Central Government. Candidades should follow all the Guidelines/Instructions regarding Covid-19. ,e-Vh-,l- inkas ij p;u gsrq NaVuh ijh{kk 8&8&2021 ¼jfookj½ dks fuEufyf[kr dsUnzkas ij vk;ksftr dh tk;sxhA vH;fFkZ;ksa dks ijh{kk gsrq cqykok i=@izos'k i= LihM ikLs V }kjk Hkst fn;s x;s gSA NaVuh ijh{kk gsrq cqyk;s x;s vH;fFkZ;ksa dh lwph vkxs i`"Bksa ij nh x;h gSA vLohd`r fd;s x;s vkosnu i=ka s dh lwph dkj.k lfgr laLFkku dh osc lkbZV ij iksLV dj nh x;h gSA vH;FkhZ mUg s vkoafVr ijh{kk dsUnz ij izkr% 7-00 cts fjikVs Z djsa rFkk ijh{kk grqs cqykok i=@izos'k i= yk;s rFkk igpku gsrq lcwr ;Fkk vk/kkj dkMZ] ernkrk igpku i=] MªkbZfoax ykbZlsUl lkFk yk;s vU;Fkk mUgs ijh{kk esa izfo"B gkus s dh vuqefr ugh nh tk;sxhA ;g Hkh /;ku dj ysosa fd NaVuh ijh{kk dk vk;kstu jkT;@dsUnz ljdkj }kjk le;≤ ij ykx w dksfoM&19 xkbZM ykbZu@vkns'kksa ds v/;k/khu gksxh ijh{kkfFkZ;ksa }kjk dksfoM+&19 ds lEcU/k esa tkjh fd;s x;s lHkh xkbZM ykbUl@fn'kk funZs'kksa dh ikyuk dh tkuh gksxhAs ROLL NO. -

A Compendium of Good Practices in Coastal and Marine Biodiversity Conservation in India
CMPA Technical Report Series 38 A Compendium of Good Practices in Coastal and Marine Biodiversity Conservation in India February 2014 Indo-German Biodiversity Programme Conservation and Sustainable Management of Coastal and Marine Protected Areas CMPA Technical Report Series No. 38 A Compendium of Good Practices in Coastal and Marine Biodiversity Conservation in India Authors Janki Teli, Sujeet Kumar Dongre, Shriji Kurup, Padma G, Rejini Simpson, Vanitha Kommu, and Reema Banerjee (Center for Environment Education) Published by Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH Indo-German Biodiversity Programme (IGBP), GIZ-India, A-2/18, Safdarjung Enclave, New Delhi - 110029, India E-Mail: [email protected] Web: www.giz.de February 2014 Responsible Director, Indo-German Biodiversity Programme Photo Credit Dr. Neeraj Khera Layout Aspire Design, Delhi Disclaimer The views expressed in this document are solely those of the authors and may not in any circumstances be regarded as stating an official position of the Ministry of Environment, Forest and Climate Change (MoEFCC), Government of India, of the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB) or the Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH. The designation of geographical entities and presentation of material in this document do not imply the expression or opinion whatsoever on the part of MoEFCC, BMUB or GIZ concerning the legal or development status of any country, territory, city or area or of its authorities or concerning the delimitation of its frontiers or boundaries. Reference herein to any specific organisation, consulting firm, service provider or process followed does not necessarily constitute or imply its endorsement, recommendation or favouring by MoEFCC, BMUB or GIZ. -

[email protected] in the Matter of FORM-I
I • J FORM-I [See Rule 8(1)] BEFORE THE NATIONAL GREEN TRIBUNAL, SOUTHERN ZONE, CHENNAI (Under Section 18(1) read with Section 14 of National Green Tribunal Act,2010) APPLICATION No. 153 of 2016 (SZ) In the Matter of 1.PARYAVARANAPARIRAKSHANA SANGHAM,Through its President Mr .Y.Krishnamurthy, Sompeta, Srikakulam District, Andhra Pradesh and another Applicants Vs 1. UNION OF INDIA Through Secretary Ministry of Environment & Forest Paryavaran Bhawan, CGO Complex, Lodhi Road, New Delhi- 110 003 and 3 others Respondents ADDITIONAL REPLY STATEMENT FILED BY 4TH RESPONDENT M/s L.G.SAHADEVAN1102/93 T-8, 4th FLOOR, SINGAPORE PLAZA, 164/337, LINGHI CHETTY STREET, CHENNAI- 600 001: PH: 9841016152 COUNSEL FOR RESPONDENT-4 [email protected] I I 1 BEFORE THE NATIONAL GREEN TRIBUNAL, SOUTHERN ZONE, CHENNAI (Under Section 18 (1)) read with Section 14 of National Green Tribunal Act, 2010) Application No.153/2016 (SZ) In the matter of 1. PARYAVARANAPARIRAKSHANA SANGHAM Through its President Mr.Y.Krishrlamurthy, Sompeta, Srikakulam District, Andhra Pradesh. · 2. E.A.S.Sarma 14-40-4 / 1, Gokhale Road, Maharanipeta, Visakhapatnam - 530002. ~ ... Applicants Versus 1. UNION OF INDIA Through Secretary Ministry of Environment & Forest ParyavaranBhawan, CGO Complex, Lodhi Road, New Delhi- 110 003. 2. CENTRALWETLANDS REGULATORY AUTHORITY, Through its Chairman Ministry of Environment and Forests And Climate Change, Indira ParayavaranBhawan, Jorbagh Road, Aliguni, New Delhi. 3. STATEOF ANDHRAPRADESH, Through its Chief Secretary, Government of Andhra Pradesh, Secretariat, Hyderabad - 500 022. 4. M/ s NCC Ltd. (formerly known asNagarjuna Construction Company Ltd). Nagarjuna Hills, Punjagutta, Hyderabad, Andhra Pradesh. (Now at NCC House, Madhapur, Hyderabad) Also at:- M/S NAGARJUNA CONSTRUCTION COMPANYLTD. -
Cyclone Preparedness & Response Plan
Government of Andhra Pradesh A P STATE DISASTER MANAGEMENT AUTHORITY REVENUE (DISASTER MANAGEMENT) DEPARTMENTCyclone Preparedness and Response Plan 0 Cyclone Preparedness and Response Plan 1 Andhra Pradesh CYCLONE PREPAREDNESS AND RESPONSE PLAN Andhra Pradesh State Disaster Management Authority Revenue (Disaster Management) Department, Govt. of Andhra Pradesh ANDHRA PRADESH STATE DISASTER MANAGEMENT AUTHORITY Genious JR Towers, D.No:21/2B, Pathuru Cross Road Centre, Kunchanpalli(PO), Tadepalli Mandal, Guntur District, Andhra Pradesh. PIN-522501. Phone / Fax: +91 8645246600 Email Id: [email protected] Technical Support, UNICEF Hyderabad Field Office Cyclone Preparedness and Response Plan 2 Cyclone Preparedness and Response Plan 3 Y.S. Jaganmohan Reddy Chief Minister of Andhra Pradesh MESSAGE I am extremely pleased to present to the people of Andhra Pradesh (AP), The Cyclone Preparedness and Response Plan, the endeavour of which is to make the state more resilient to Cyclones. It will definitely help to enhance the ability of the state to cope up with the hazard at its all levels of intensity by integrating disaster risk reduction measures in all developmental concerns and improved mitigation and preparedness strategies. The natural hazard events are beyond our control, but our efforts to reduce risks from hazards by building our capability in the areas of preparedness, response and recover from the losses has improved significantly. We have considerably enhanced our technical know-how in forecasting and closely monitoring hazards like Cyclones. Nevertheless, we still have to make our disaster management system to rank among the very best in the country. I commend the AP-SDMA to embark on a comprehensive plan; and thereby, integrating mitigation, preparedness and response measures. -

Srikakulam Urban Development Authority (SUDA) with Head Quarters at Srikakulam – Notification - Orders – Issued
GOVERNMENT OF ANDHRA PRADESH A B S T R A C T Municipal Administration & Urban Development Department – The Andhra Pradesh Metropolitan Region and Urban Development Authorities Act,2016 (Act No. 5 of 2016) – Constitution of Srikakulam Urban Development Authority (SUDA) with Head Quarters at Srikakulam – Notification - Orders – Issued. MUNICIPAL ADMINISTRATION & URBAN DEVELOPMENT (H) DEPARTMENT G.O.MS.No. 63 Dated: 12-02-2019 Read the following: 1. The Andhra Pradesh Metropolitan Region and Urban Development Authorities Act, 2016 (Act No. 5 of 2016) 2. G.O.Ms. No.26 MA&UD (H) Department, dated 08.02.2016. 3. From the Director of Town and Country Planning, Andhra Pradesh, No.2920/16/P, dated 30.01.2019 and 05.02.2019 4. G.O.Ms.No.62,MA&UD (H) Department, dated 12.02.2019 & & & O R D E R: The Andhra Pradesh Metropolitan Region and Urban Development Authorities Act, 2016 (Act No. 5 of 2016) has come into force w.e.f 08.02.2016 by virtue of notification issued in G.O. 2nd read above. 2. In the reference 3rd read above, the Director of Town & Country Planning, AP, Guntur has requested the Government for constitution of Srikakulam Urban Development Authority (SUDA), with a jurisdiction of 19 Mandals covering 968 villages and three (03) Urban Local Bodies i.e., Ichapuram, Palakonda and Palasa-Kasibugga Municipalities with an extent of 2542.53 Sq.Kms and population of 12,57,599 as per 2011 census with Head Quarters at Palasa-Kasibugga under Sec. 3 & 4, Chapter-II of Andhra Pradesh Metropolitan Region and Urban Development Authorities Act, 2016.