The Effect of L-Ornithine Hydrochloride Ingestion on Performance During
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of a System of High Ornithine and Citrulline Production by a Plant-Derived Lactic Acid Bacterium, Weissella Confusa K-28
Development of a system of high ornithine and citrulline production by a plant-derived lactic acid bacterium, Weissella confusa K-28 㸦᳜≀⏤᮶ங㓟⳦ :HLVVHOODFRQIXVD. ࠾ࡅࡿ ࢜ࣝࢽࢳࣥཬࡧࢩࢺࣝࣜࣥ㧗⏕⏘ࢩࢫࢸ࣒ࡢᵓ⠏㸧 Md Rakhimuzzaman Student ID: D164180 Supervisor: Prof. Masanori Sugiyama Department of Probiotic Science for Preventive Medicine Graduate School of Biomedical and Health Sciences Hiroshima University, Japan 2019 ABBREVIATIONS ADI arginine deiminase AUC area under curve BLAST basic local alignment search tool CK carbamate kinase dNTP deoxyribonucleotide triphosphate DDBJ DNA Data Bank of Japan EDTA ethylenediamine tetra-acetic acid EtOH ethanol EPS exopolysaccharide GABA gamma-aminobutyric acid GRAS generally recognized as safe HPLC high performance liquid chromatography LAB lactic acid bacteria Lb. Lactobacillus OD optical density OTC ornithine carbamoyltransferase PITC phenyl isothiocyanate TAE tris-acetate-ethylenediamine tetra-acetic acid TE tris-ethylenediamine tetra-acetic acid TEA triethylamine Tris tris(hydroxymethyl)aminomethane rDNA ribosomal deoxyribonucleic acid RT-PCR reverse transcription polymerase chain reaction CONTENTS INTRODUCTION 1-17 OBJECTIVES 18 MATERIALS AND METHODS 19-34 RESULTS 35-57 DISCUSSIONS 58-65 CONCLUSION 66-67 ACKNOWLEDGEMENTS 68 REFERENCES 69-84 INTRODUCTION Lactic acid bacteria (LAB) are an order of gram-positive, acid-tolerant, generally nonsporulating, non-respiring, either rod-shaped or spherical microorganisms. Since LAB lack the ability of synthesizing porphyrins (heme, and components of respiratory chains) (Pessione et al, 2010), the bacteria can not generate ATP without external heme supplementation (Pessione et al, 2010). The LAB strains can only obtain ATP by fermentation, usually they generate the ATP from some sugars. Because LAB do not use oxygen for energy production, the bacteria easily grow under anaerobic conditions, but they can also grow in presence of oxygen. -
Dietary Supplements Compendium 2019 Edition
Products and Services New Dietary Supplements Reference Standards Below is a list of newly released Reference Standards. Herbal Medicines/ Botanical Dietary Supplements Baicalein Baicalein 7-O-Glucuronide Chebulagic Acid Dietary Supplements Compendium 2019 Edition Coptis chinensis Rhizome Dry Extract In response to the customer feedback, coupled with evolving information needs, USP moved the Psoralen Dietary Supplements Compendium (DSC) to an online platform for the 2019 edition. DSC continues Scutellaria baicalensis Root Dry to provide in-depth, comprehensive information for all phases of development and manufacturing of Extract quality dietary supplements including quality control, quality assurance, and regulatory/compendial Terminalia chebula Fruit Dry affairs. Extract Guarana Seed Dry Extract Some of the advantages that come with the new online DSC edition include: Cullen Corylifolium Fruit Dry Extract More frequent updates to ensure access to the most current information Procyanidin B2 Customizable alerts to notify of changes to selected documents An intuitive interface to facilitate quick and easy navigation Non-Botanicals A customizable workspace with bookmarks, alerts and a viewing history beta-Glycerylphosphorylcholine Convenient, anytime, anywhere access with common browsers Conjugated Linoleic Acids – In addition to selected new and revised monographs and General Chapters from the USP-NF and Triglycerides Food Chemicals Codex issued since the previous 2015 edition, the DSC 2019 features: Creatine Docosahexaenoic Acid 24 new General Chapters Eicosapentaenoic Acid 72 new dietary ingredient and dietary supplement monographs L-alpha- 27 sets of supplementary information for botanical and nonbotanical dietary supplements Glycerophosphorylethanolamine 59 updated botanical HPTLC plates L-alpha- Revised and updated dietary intake comparison tables Glycerylphosphorylcholine Updated Dietary Supplement Verification Program manual Omega-3 Free Fatty Acids Pyrroloquinoline Quinone View this page for more information or to subscribe to the 2019 online DSC. -

Metabolic Reprogramming in Prostate Cancer
www.nature.com/bjc REVIEW ARTICLE Metabolic reprogramming in prostate cancer Fahim Ahmad 1,2, Murali Krishna Cherukuri2 and Peter L. Choyke1 Although low risk localised prostate cancer has an excellent prognosis owing to effective treatments, such as surgery, radiation, cryosurgery and hormone therapy, metastatic prostate cancer remains incurable. Existing therapeutic regimens prolong life; however, they are beset by problems of resistance, resulting in poor outcomes. Treatment resistance arises primarily from tumour heterogeneity, altered genetic signatures and metabolic reprogramming, all of which enable the tumour to serially adapt to drugs during the course of treatment. In this review, we focus on alterations in the metabolism of prostate cancer, including genetic signatures and molecular pathways associated with metabolic reprogramming. Advances in our understanding of prostate cancer metabolism might help to explain many of the adaptive responses that are induced by therapy, which might, in turn, lead to the attainment of more durable therapeutic responses. British Journal of Cancer https://doi.org/10.1038/s41416-021-01435-5 BACKGROUND Several tools have been designed to assess metabolism without Cellular metabolism involves complex biochemical processes disturbing the system. Advances in nuclear magnetic resonance through which specific nutrients are consumed. Carbohydrates, and mass spectroscopy have helped to quantify the carbon influx fatty acids and amino acids are the main source of nutrients for of the central metabolic pathways (e.g. TCA cycle, glycolysis and energy homeostasis and macromolecular synthesis. They are the pentose phosphate pathway) in mammalian systems, and this main constituent of core metabolic pathways that can be approach has been instrumental in demonstrating that the classified as anabolic, catabolic or waste producing (Fig. -

The Diagnosis and Management of Ornithine Transcarbamylase Deficiency in Pregnancy: a Case Report
Perinatal Journal 2011;19(1):23-27 e-Adress: http://www.perinataljournal.com/20110191006 doi:10.2399/prn.11.0191006 The Diagnosis and Management of Ornithine Transcarbamylase Deficiency in Pregnancy: A Case Report Orkun Çetin1, Cihat fien1, Begüm Aydo¤an1, Seyfettin Uluda¤1, ‹pek Dokurel Çetin2, Hakan Erenel1 1Cerrahpafla T›p Fakültesi Kad›n Hastal›klar› ve Do¤um Anabilim Dal›, ‹stanbul, Türkiye 2Cerrahpafla T›p FakültesiÇocuk Sa¤l›¤› ve Hastal›klar› Anabilim Dal›, ‹stanbul Türkiye Abstract Objective: Ornithine transcarbamylase (OTC) deficiency is the most common urea cycle disorder. In our case, we discussed the fol- low up and the management of the OTC deficiency patient, diagnosed during pregnancy. Case: 32-year-old patient, OTC deficiency was diagnosed during pregnancy which was resulted with missed abortion. In the next pregnancy, the patient treated with phenyl butyrate, arginin ornitin lisin and carbamazepine. Coryon villus sampling (CVS) was done in the first trimester. There was not any mutation in the fetal gene locus. In the 39. gestational week, healthy female baby was deliv- ered by caesarean section. Conclusion: OTC deficiency is a rare disease. To make the followup and the management of these patients during pregnancy, may require knowledge and experience about complications. The treatment must be carried out with multidisciplinary approach. The genetic counseling should be given to the family about the prenatal diagnosis of OTC deficiency (CVS, amniosentesis). Keywords: Ornithine transcarbamylase deficiency, pregnancy, prenatal diagnosis, multidisciplinary approach. Gebelikte ornitin transkarbamilaz eksikli¤i tan›s› ve yönetimi: Olgu sunumu Amaç: Ornitin transkarbamilaz (OTC) eksikli¤i, en s›k rastlanan üre döngüsü bozuklu¤udur. -

Amino Acid Degradation; Urea Cycle
Amino acid degradation; urea cycle Kristina Mlinac Jerković [email protected] In animals, amino acids undergo oxidative degradation in three different metabolic circumstances: 1. During the normal synthesis and degradation of cellular proteins, some amino acids that are released from protein breakdown and are not needed for new protein synthesis undergo oxidative degradation. 2. When a diet is rich in protein and the ingested amino acids exceed the body’s needs for protein synthesis, the surplus is catabolized; amino acids cannot be stored. 3. During starvation or in uncontrolled diabetes mellitus, when carbohydrates are either unavailable or not properly utilized, cellular proteins are used as fuel. Metabolic fates of amino groups - The metabolism of nitrogen-containing molecules such as proteins and nucleic acids differs significantly from that of carbohydrates and lipids. - Whereas the latter molecules can be stored and mobilized as needed for biosynthetic reactions or for energy generation, there is no nitrogen-storing molecule (one exception to this rule is storage protein in seeds). - Organisms must constantly replenish their supply of usable nitrogen to replace organic nitrogen that is lost in catabolism. For example, animals must have a steady supply of amino acids in their diets to replace the nitrogen excreted as urea, uric acid, and other nitrogenous waste products. - Since excess dietary amino acids are neither stored for future use nor excreted, they are converted to common metabolic intermediates such as pyruvate, oxaloacetate, acetyl-CoA, and α-keto-glutarate. - Consequently, amino acids are also precursors of glucose, fatty acids, and ketone bodies and are therefore metabolic fuels. -

Characterization of High-Ornithine-Producing Weissella Koreensis DB1 Isolated from Kimchi and Its Application in Rice Bran Fermentation As a Starter Culture
foods Article Characterization of High-Ornithine-Producing Weissella koreensis DB1 Isolated from Kimchi and Its Application in Rice Bran Fermentation as a Starter Culture Mun So Yeong , Moon Song Hee and Chang Hae Choon * Kimchi Research Center, Department of Food and Nutrition, Chosun University, 309 Pilmun-daero, Dong-gu, Gwangju 61452, Korea; [email protected] (M.S.Y.); [email protected] (M.S.H.) * Correspondence: [email protected] Received: 11 September 2020; Accepted: 23 October 2020; Published: 26 October 2020 Abstract: High-ornithine-producing Weissella koreensis DB1 were isolated from kimchi. Ornithine is produced from arginine via the intracellular arginine deiminase pathway in microorganisms; thus, high cell growth is important for producing ornithine in large quantities. In this study, excellent W. koreensis DB1 growth (A600: 5.15–5.39) was achieved in de Man, Rogosa, and Sharpe (MRS) medium supplemented with 1.0–3.0% arginine (pH 5.0) over 24–48 h at 30 ◦C, and the highest ornithine (15,059.65 mg/L) yield was obtained by culture in MRS containing 3.0% arginine for 48 h. W. koreensis DB1 was further investigated as a functional starter culture for rice bran fermentation. After 48 h of fermentation at 30 ◦C, the fermented rice bran was freeze-dried and ground. The prepared fermented rice bran contained 43,074.13 mg/kg of ornithine and 27,336.37 mg/kg of citrulline, which are used as healthcare supplements due to their beneficial effects. Furthermore, the organoleptic quality of the fermented rice bran was significantly improved, and the fermented product contained viable cells (8.65 log CFU/mL) and abundant dietary fiber. -

Protein Metabolism and It's Disorders
Protein Metabolism and it’s Disorders (Course : M.Sc. Zoology) Course Code: ZOOL 4008 (Biochemistry and Metabolism), Semester –II Dr. Shyam Babu Prasad Assistant Professor Department of Zoology Mahatma Gandhi Central University (MGCU), Motihari-845401 (Bihar) Email: [email protected] Important facts of the Protein Metabolism •Proteins metabolism referred as synthesis of protein from Amino acids (Anabolism) and breakdown of proteins (Catabolism). •In the Unit II, we discussed the synthesis (Anabolism) of various form of the proteins from different amino acids. • Here we mainly focused on the Catabolism of the protein, which is integral part of metabolism of the nitrogen-containing molecules. • Nitrogen enters in to the body in various form of compounds that is present in food, the most important is amino acids (as dietary food). • During Protein/amino acid metabolism (Catabolism), Nitrogen leaves the body as urea, ammonia, and in form of other derivatives. • The Catabolism of the protein depends on the amino acid pool and protein turnover (the rate of protein synthesis and degradation is equal). • The amino acids present in through out of the body (cells, blood, and the extracellular fluids etc) is called amino acid pools. It is maintained by following factors as represented in below diagram: Body protein Dietary degradation Protein 400gm/day 600gm/day Supplied Amino acid pool Depleted Synthesis of Remaining Synthesis of Glucose, Synthesis of amino acids Nitrogen containing Glycogen, the Body burned as compound like Ketone Proteins -
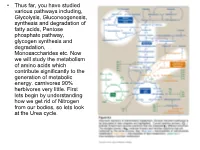
Transaminations and Urea Cycle
• Thus far, you have studied various pathways including, Glycolysis, Gluconeogenesis, synthesis and degradation of fatty acids, Pentose phosphate pathway, glycogen synthesis and degradation, Monosaccharides etc. Now we will study the metabolism of amino acids which contribute significantly to the generation of metabolic energy. carnivores 90% herbivores very little. First lets begin by understanding how we get rid of Nitrogen from our bodies, so lets look at the Urea cycle. 1 α Amino group keeps amino acids safe from oxidative breakdown. Removal of this group is essential for energy production it is an obligatory step for catabolism. Nitrogen can then be incorporated or excreted.This is done through transamination or oxidative deamination, provide amonia and aspartate sources for the Urea cycle. Transamination is funneled through glutamate. This is done through the transfer of the α amino group to α ketoglutarate. Product α-keto acid and glutamate. Alanine to pyruvate and aspartate to oxaloacetate all amino acids except lysine and threonina (lose the α amino through deamination. Glutamate dehydrogenase the oxidative deamination Glutamate only amino acid that undergoes rapid oxidative deamination (glutamate dehydrogenase NH3 + NADH), where amino groups are released as ammonia. After a meal rich in protein the levels of glutamate in liver are increased favoring amino acid degradation and formation of ammonia. Activators of this enzyme are ADP and GDP while ATP and GTP are inhibitors. This reaction occurs in the mitochondria. Ammonia can be transported to the liver from other tissues through two mechanisms that reduce the toxicity of ammonia. One through Glutamine (most tissues through, glutamine synthetase). In the liver the glutamine is converted back to glutamate (Fig. -

ATP Structure and Function
ATP: Universal Currency of Cellular Energy All living things including plants, animals, birds, insects, humans need energy for the proper functioning of cells, tissues and other organ systems. As we are aware that green plants, obtain their energy from the sunlight, and animals get their energy by feeding on these plants. Energy acts as a source of fuel. We, humans, gain energy from the food we eat, but how are the energy produced and stored in our body. A living cell cannot store significant amounts of free energy. Excess free energy would result in an increase of heat in the cell, which would result in excessive thermal motion that could damage and then destroy the cell. Rather, a cell must be able to handle that energy in a way that enables the cell to store energy safely and release it for use only as needed. Living cells accomplish this by using the compound adenosine triphosphate (ATP). ATP is often called the “energy currency” of the cell, and, like currency, this versatile compound can be used to fill any energy need of the cell. How? It functions similarly to a rechargeable battery. When ATP is broken down, usually by the removal of its terminal phosphate group, energy is released. The energy is used to do work by the cell, usually by the released phosphate binding to another molecule, activating it. For example, in the mechanical work of muscle contraction, ATP supplies the energy to move the contractile muscle proteins. Recall the active transport work of the sodium-potassium pump in cell membranes. -

Laboratory Diagnostic Approaches in Metabolic Disorders
470 Review Article on Inborn Errors of Metabolism Page 1 of 14 Laboratory diagnostic approaches in metabolic disorders Ruben Bonilla Guerrero1, Denise Salazar2, Pranoot Tanpaiboon2,3 1Formerly Quest Diagnostics, Inc., Ruben Bonilla Guerrero, Rancho Santa Margarita, CA, USA; 2Quest Diagnostics, Inc., Denise Salazar and Pranoot Tanpaiboon, San Juan Capistrano, CA, USA; 3Genetics and Metabolism, Children’s National Rare Disease Institute, Washington, DC, USA Contributions: (I) Conception and design: All authors; (II) Administrative support: R Bonilla Guerrero; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Ruben Bonilla Guerrero. Formerly Quest Diagnostics, Inc., Ruben Bonilla Guerrero, 508 Sable, Rancho Santa Margarita, CA 92688, USA. Email: [email protected]. Abstract: The diagnosis of inborn errors of metabolism (IEM) takes many forms. Due to the implementation and advances in newborn screening (NBS), the diagnosis of many IEM has become relatively easy utilizing laboratory biomarkers. For the majority of IEM, early diagnosis prevents the onset of severe clinical symptoms, thus reducing morbidity and mortality. However, due to molecular, biochemical, and clinical variability of IEM, not all disorders included in NBS programs will be detected and diagnosed by screening alone. This article provides a general overview and simplified guidelines for the diagnosis of IEM in patients with and without an acute metabolic decompensation, with early or late onset of clinical symptoms. The proper use of routine laboratory results in the initial patient assessment is also discussed, which can help guide efficient ordering of specialized laboratory tests to confirm a potential diagnosis and initiate treatment as soon as possible. -

Tri-Amino™ Support for Healthy Function of Muscle and Vascular Systems
PRODUCT DATA DOUGLAS LABORATORIES® 04/2012 1 Tri-Amino™ Support for healthy function of muscle and vascular systems DESCRIPTION Tri-Amino™, provided by Douglas Laboratories, includes significant amounts of the amino acids L-ornithine, L-arginine, and L-lysine. FUNCTIONS Amino acids have many functions in the body. They are the building blocks for all body proteins—structural proteins that build muscle, connective tissues, bones and other structures, and functional proteins in the form of thousands of metabolically active enzymes. Amino acids provide the body with the nitrogen that is essential for growth and maintenance of all tissues and structures. Aside from these general functions, individual amino acids also have specific functions in many aspects of human physiology and biochemistry. L-arginine is a conditionally essential dibasic amino acid. The body is usually capable of producing sufficient amounts of arginine, but in times of physical stress, endogenous synthesis is often inadequate to meet the increased demands. L-arginine can either be used for glucose synthesis or catabolized to produce energy via the tricarboxylic acid cycle. It is also the sole source of nitric oxide (NO), via the enzyme nitric oxide synthase. NO can affect a variety of physiological processes, including relaxation of arterial smooth muscle, platelet aggregation, and neuroendocrine secretion. L-arginine is required for the synthesis of creatine phosphate. Similar to adenosine triphosphate (ATP), creatine phosphate functions as a carrier of readily available energy for contractile work in muscles. Adequate reservoirs of creatine phosphate are necessary in muscle as an energy reserve for anaerobic activity. L-arginine is also a precursor of polyamines, including putrescine, spermine and spermidine. -

Serum Metabolite Profiles As Potential Biochemical Markers in Young
www.nature.com/scientificreports OPEN Serum metabolite profles as potential biochemical markers in young adults with community- acquired pneumonia cured by moxifoxacin therapy Bo Zhou1, Bowen Lou2,4, Junhui Liu3* & Jianqing She2,4* Despite the utilization of various biochemical markers and probability calculation algorithms based on clinical studies of community-acquired pneumonia (CAP), more specifc and practical biochemical markers remain to be found for improved diagnosis and prognosis. In this study, we aimed to detect the alteration of metabolite profles, explore the correlation between serum metabolites and infammatory markers, and seek potential biomarkers for young adults with CAP. 13 Eligible young mild CAP patients between the ages of 18 and 30 years old with CURB65 = 0 admitted to the respiratory medical department were enrolled, along with 36 healthy participants as control. Untargeted metabolomics profling was performed and metabolites including alcohols, amino acids, carbohydrates, fatty acids, etc. were detected. A total of 227 serum metabolites were detected. L-Alanine, 2-Hydroxybutyric acid, Methylcysteine, L-Phenylalanine, Aminoadipic acid, L-Tryptophan, Rhamnose, Palmitoleic acid, Decanoylcarnitine, 2-Hydroxy-3-methylbutyric acid and Oxoglutaric acid were found to be signifcantly altered, which were enriched mainly in propanoate and tryptophan metabolism, as well as antibiotic-associated pathways. Aminoadipic acid was found to be signifcantly correlated with CRP levels and 2-Hydroxy-3-methylbutyric acid and Palmitoleic acid with PCT levels. The top 3 metabolites of diagnostic values are 2-Hydroxybutyric acid(AUC = 0.90), Methylcysteine(AUC = 0.85), and L-Alanine(AUC = 0.84). The AUC for CRP and PCT are 0.93 and 0.91 respectively.