Vaccines and Global Health :: Ethics and Policy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protection in Danger
Protection in Danger Monthly News Brief Monthly News Brief October Safety, security and access incidents 2019 Incidents of threats and violence in refugee and IDP settings Africa This monthly digest Nigeria comprises threats and 05 October 2019: In Banki town, Bama district, Borno state, Boko incidents of violence Haram militants stormed an unnamed IDP camp, killing two IDPs and affecting protection of injuring three guards. Source: Sahara Reporters IDPs and refugees. South Sudan 15 October 2019: In Central Equatoria state, an IDP was shot and killed It is prepared by Insecurity and another injured outside of the UN PoC site by pastoralist militia. Insight from information available in open sources. The dispute is believed to be related to harassment of women. Sources: ACLED1 and Radio Tamazuj Visit our website to download Sudan previous Protection in Danger 02 October 2019: In Kabkabiya district, North Darfur state, two soldiers Monthly News Briefs. raped a teenage girl near the Sortony IDP camp. Sources: Dabanga Sudan I and Dabanga Sudan II Access data from the Protection in Danger Monthly News Brief 02 October 2019: Near Nabagai, 5km west of Gereida, South Darfur on HDX Insecurity Insight. state, three IDP farmers were attacked by gunmen, one was killed and the others wounded after they resisted the efforts of the attackers to steal their donkey cart. Source: ACLED1 Join our mailing list to receive monthly reports on insecurity 05 October 2019: Near Shalaiya IDP camp, Nierteti, Central Darfur affecting the protection of IDPs and refugees. state, two teenage girls believed to be IDPs were raped by five armed pastoralists in military uniform. -

Vaccines and Global Health :: Ethics and Policy
Vaccines and Global Health: The Week in Review 28 October 2017 Center for Vaccine Ethics & Policy (CVEP) This weekly digest targets news, events, announcements, articles and research in the vaccine and global health ethics and policy space and is aggregated from key governmental, NGO, international organization and industry sources, key peer-reviewed journals, and other media channels. This summary proceeds from the broad base of themes and issues monitored by the Center for Vaccine Ethics & Policy in its work: it is not intended to be exhaustive in its coverage. Vaccines and Global Health: The Week in Review is also posted in pdf form and as a set of blog posts at https://centerforvaccineethicsandpolicy.net. This blog allows full-text searching of over 8,000 entries. Comments and suggestions should be directed to David R. Curry, MS Editor and Executive Director Center for Vaccine Ethics & Policy [email protected] Request an email version: Vaccines and Global Health: The Week in Review is published as a single email summary, scheduled for release each Saturday evening before midnight (EST/U.S.). If you would like to receive the email version, please send your request to [email protected]. Support this knowledge-sharing service: Your financial support helps us cover our costs and to address a current shortfall in our annual operating budget. Click here to donate and thank you in advance for your contribution. Contents [click on link below to move to associated content] A. Milestones :: Perspectives :: Featured Journal Content B. Emergencies: Polio; Zika; Ebola/EVD; MERS-Cov; Yellow Fever C. -

Situation Report: WHO Syria, Week 19-20, 2019
WHO Syria: SITUATION REPORT Weeks 28 – 29 (5 – 18 July), 2019 I. General Development, Political and Security Situation (22 June - 4July), 2019 The security situation within the country remains volatile and unstable. The main hot spots remain Daraa, Al- Hassakah, Deir Ezzor, Latakia, Hama, Aleppo and Idlib governorates. The Turkish military preparations along the Syrian /Turkish borders escalated the tension in the Euphrates region ushering in an imminent military operation against the Kurds. The Eastern governorates are still witnessing a high level of asymmetric attacks against SDF personnel in the form of of IEDs and VBIEDs explosions. The security situation in North rural Hama remained tense; SAA regained control over a town that was seized by NSAGs a week ago . Military operations are still taking place against NSAGs held towns in Idlib, Hama, Latakia and Aleppo Governorates. An increase in the number of Indirect Artillery Fire attack (AIF) has been noted in Aleppo city in comparison with the previous week. At least five Syrian soldiers were killed after being attacked in the governorate of Daraa, 90 km south of the capital Damascus. Military sources asserted that the terrorists ambushed a military vehicle between Yadouda and Dahya, leaving five soldiers dead and 16 injured. Air strikes targeted rebel-held cities in northwest Syria on Friday, a war monitor reported, widening bombardment of the last major insurgent enclave to areas that had mostly escaped it. The strikes killed three people in Idlib and three in Maarat al-Numan, two of the largest cities in the region, the Britain-based Syrian Observatory for Human Rights said according to a Reuters report. -

Syria Cvdpv2 Outbreak Situation Report 39, 31 May 2018
Syria cVDPV2 outbreak Situation Report # 39 May 2018 issue Distribution of cVDPV2 cases in Deir Ez-Zor, Raqqa and Homs governorates, Summary Syria, 2017 and 2018 New cVDPV2 cases this week: 0 Total number of cVDPV2 cases: 74 Outbreak grade: 3 Infected governorates and districts Number of Governorate District cVDPV2 cases to date Deir Ez-Zor Mayadeen 58 Deir Ez-Zor 1 Boukamal 12 Raqqa Tell Abyad 1 Thawra 1 Homs Tadmour 1 Index case Location: Mayadeen district, Deir Ez-Zor governorate Onset of paralysis: 3 March 2017, age: 22 months, vac- cination status: 2 OPV/zero IPV Most recent case (by date of onset) Location: Boukamal, Deir Ez-Zor governorate The boundaries and names shown and the designations used on this map do not imply official endorsement or Onset of paralysis: 21 September 2017, age: 5 months, acceptance by the United Nations. Source: Syrian Arab Republic, Administrative map, DFS, United Nations 2012 vaccination status: zero OPV/zero IPV Key highlights Characteristics of the cVDPV2 cases Median age: 15 months No new cases of cVDPV2 have been reported in 2018. The most recent Gender: approximately two thirds of cases are female case (by date of onset of paralysis) is 21 September 2017 from Boukamal Vaccination status of the cases: district, Deir Ez-Zor governorate. The total number of cVDPV2 cases - IPV: 11 cases (15%) received IPV remains 74. - OPV: 41% zero dose, 42% have received 1-2 doses An independent outbreak response assessment (OBRA) of the cVDPV2 Distribution of non-polio AFP (NPAFP) and circulating vaccine-derived poliovirus type-2 (cVDPV2), Deir Ez- outbreak in Syria concluded in April. -

Syria Cvdpv2 Outbreak Situation Report # 5 18 July 2017
Syria cVDPV2 outbreak Situation Report # 5 18 July 2017 cVDPV2 cases in Deir Ez-Zor and Raqqa governorates, Syria, 2017 Summary New cVDPV2 cases this week: 4 Total number of cVDPV2 cases: 27 Outbreak grade: 3 Index case Onset of paralysis: 3 March 2017 Location: Mayadeen district, Deir Ez-Zor Age: 22 months Vaccination status: 2 OPV doses/zero IPV Most recent cases Mayadeen district, Deir Ez-Zor Onset of paralysis: 6 June 2017, age: 8 months, vaccination status: 1 OPV/zero IPV Tell Abyad district, Raqqa Onset of paralysis: 25 April 2017, age: 97 months, vaccination status: 3 OPV/1 IPV Affected districts Mayadeen and Tell Abyad districts Immunization response The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Two rounds planned for Deir Ez-Zor and Nations. Source: Syrian Arab Republic, Administrative map, DFS, United Nations 2012 Raqqa. First rounds in both governorates Key highlights are expected to commence 22 July. Twenty-seven (27) cases of circulating vaccine-derived poliovirus type-2 Characteristics of the cVDPV2 cases (cVDPV2) have been confirmed in Syria —26 cases are from Mayadeen district, Median age: 15 months Deir Ez-Zor governorate, and 1 case from Tell Abyad district, Raqqa Gender ratio male-female: 1:2 governorate. This is an increase of 4 cases, since the last situation report dated Vaccination status of the cases: 12 July. The most recent case (Mayadeen district) had onset of paralysis on 6 · IPV: 4 cases (15%) received IPV June. · OPV: 30% zero dose, 48% have received 1-2 doses Monovalent type-2 oral polio vaccine (mOPV2) reached Deir Ez-Zor on 12 July. -

The PYD's Separatist Project
The PYD’s Separatist Project in the Syrian Euphrates Region Abdullah Al-Najjar Political research Hermon Center for Contemporary Studies is a non-profit organization, focused mainly on producing studies and research on the Syrian situation, implementing and managing projects, activities and initiatives to rebuild Syria on the foundations of democracy, freedom, social justice, human rights, human dignity and equal citizenship values. For Contacts: e-mail: [email protected] Harmoon Center For Contemporary Studies The PYD’s Separatist Project in the Syrian Euphrates Region January 2020 Abdullah Al-Najjar Authors This study was conducted by Abdullah Al-Najjar, with the help of former col- leagues and acquaintances who assisted him in collecting and verifying the infor- mation. Three of them had submitted three background papers: two on education, and one on the oil issue. In light of the positions of these individuals where they live, we will not be disclosing their names, but would like to thank them very much. Abdullah Al-Najjar is a former officer at the Political Security Directorate, with a degree in law. He worked in Hasakeh Province for 12 years, including nine and a half years in Qamishli district, as an assistant and head of the Qamishli police station, and head of the Amuda police station. He defected from the Political Se- curity Directorate in 2012 with the rank of major. He currently works in the field of studies and research related to east of the Euphrates, and on issues related to security and the military. Harmoon Center For Contemporary -

WHO Syria: SITUATION REPORT Weeks 24 – 25 (07 - 21 June), 2019
WHO Syria: SITUATION REPORT Weeks 24 – 25 (07 - 21 June), 2019 I. General Development, Political and Security Situation ✓ The security situation within Syria remains unstable and volatile; the main hot spots being, Hama, Idlib, Aleppo and Ar-Raqqah. SAA aerial and ground shelling are still taking place against NSAGs positions along the frontline axis in North rural Hama and South rural Idlib. Shelling and counter shelling continue between the Turkish Army and Kurdish forces in North rural Aleppo. ✓ A cease-fire between Syrian government forces and the opposition brokered by Russia and Turkey on 12 June appeared at risk within hours of being declared as Ankara accused President Bashar al-Assad’s forces of attacking a Turkish military outpost. Reports of heavy fighting, airstrikes and artillery shelling on several fronts in northern rural Hama governorate continued following the announcement. As of 16 June, the ceasefire had not been implemented. ✓ On 17 June, Saudi Crown Prince succumbed to Washington and announced his country's agreement with US aggressive plans in Syria ... one day after a Saudi minister sneaked to areas under control Syrian Democratic Forces (SDF), where he offered his country's support to SDF separatist aspirations and met with US officials and SDF-allied tribal leaders. ✓ With the six-month reconciliation ‘grace period’ in Dar’a governorate set to expire on 24 June, the overall situation in the southern governorates is growing increasingly tense. A few new checkpoints were established in Jlein, Masaken Jlein, Sahm El Golan, and Mzerea in Yarmouk basin. NSAGs announced the formation of a new military group called “Popular Resistance” and launched several attacks against the newly established checkpoints, which resulted in several deaths and injuries on both sides ✓ At least 10 people have been killed by fires engulfing vital wheat fields across Syria's northeast, according to a UK- based activist group, as Kurdish authorities claimed the fields had been set on fire deliberately. -
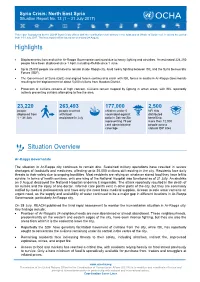
Ar Raqqa Sit Rep July No 13.Pdf
Syria Crisis: North East Syria Situation Report No. 13 (1 – 31 July 2017) This report is produced by the OCHA Syria Crisis offices with the contribution of all sectors in the hubs and at Whole of Syria level. It covers the period from 1-31 July 2017. The next report will be issued on or around 24 August. Highlights Displacements from and within Ar-Raqqa Governorate continued due to heavy fighting and airstrikes. An estimated 224,250 people have been displaced since 1 April, including 45,486 since 1 June. Up to 25,000 people are estimated to remain inside Raqqa city, amid heavy fighting between ISIL and the Syria Democratic Forces (SDF). The Government of Syria (GoS) and aligned forces continued to clash with ISIL forces in southern Ar-Raqqa Governorate resulting to the displacement of about 5,000 civilians from Maadan District. Protection of civilians remains of high concern. Civilians remain trapped by fighting in urban areas, with ISIL reportedly actively preventing civilians attempting to flee the area. 23,220 263,493 177,000 2,500 people people reached children under 5 NFI Kits displaced from with food vaccinated against distributed 1 – 31 July assistance in July polio in Deir-ez-Zor benefiting representing 79 per more than 12,000 cent administrative people across coverage various IDP sites Situation Overview Ar-Raqqa Governorate The situation in Ar-Raqqa city continues to remain dire. Sustained military operations have resulted in severe shortages of foodstuffs and medicines, affecting up to 25,000 civilians still residing in the city. Residents face daily threats to their safety due to ongoing hostilities. -

Syria Cvdpv2 Outbreak Situation Report # 20 31 October 2017
Syria cVDPV2 outbreak Situation Report # 20 31 October 2017 cVDPV2 cases in Deir Ez-Zor, Raqqa and Homs governorates, Syria, 2017 Summary New cVDPV2 cases this week: 1 Total number of cVDPV2 cases: 53 Outbreak grade: 3 Infected governorates and districts Number of Governorate District cVDPV2 cases to date Deir Ez-Zor Mayadeen 43 Deir Ez-Zor 1 Boukamal 6 Raqqa Tell Abyad 1 Thawra 1 Homs Tadmour 1 Index case Location: Mayadeen district, Deir Ez-Zor governorate Onset of paralysis: 3 March 2017, age: 22 months, vaccination status: 2 OPV doses/zero IPV Most recent case (by date of onset) Location: Mayadeen, Deir Ez-Zor governorate Onset of paralysis: 25 August , age: 9 months, The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Nations. Source: Syrian Arab Republic, Administrative map, DFS, United Nations 2012 vaccination status: 1 OPV/zero IPV Characteristics of the cVDPV2 cases Key highlights Median age: 16 months One (1) new case of cVDPV2 was reported this week from Mayadeen, Deir Gender ratio male-female: 3:5 Ez-Zor governorate. The date of onset of the case was 18 August 2017. The Vaccination status of the cases: - IPV: 10 cases (19%) received IPV most recent case (by date of onset) remains 25 August - OPV: 32% zero dose, 47% have received 1-2 The total number of cVDPV2 cases is 53 doses Third party independent monitoring results for the second outbreak response round for Raqqa governorate have been received. Reported Distribution of non-polio AFP (NPAFP) and circu- lating vaccine-derived poliovirus type-2 (cVDPV2), coverage of targeted children is 69% (measured by parental recall through a Deir Ez-Zor, Raqqa and Homs governorates, 2017 house to house survey). -

Ar-Raqqa Crisis Overview: November 2016 - October 2017 Syria, November 2017
Ar-Raqqa Crisis Overview: November 2016 - October 2017 Syria, November 2017 EXECUTIVE SUMMARY Map 1: Ar-Raqqa Governorate and Sub-districts1 Between November 2016 and October 2017, the campaign to expel the group known as the Islamic State of Iraq and the Levant (ISIL) from Ar-Raqqa governorate led to an escalation of TURKEY conflict across the area. Conflict resulted in multiple phases of significant displacement of civilians ² TELL ABIAD and resulted in high levels of need of both the displaced and those that remained in areas of direct ALEPPO conflict. Furthermore, the ability of host communities across Ar-Raqqa governorate to absorb internally displaced persons (IDPs) became increasingly stretched as their number grew. With EIN ISSA SULUK AL-HASAKEH conflict reducing across the governorate at the time of writing, thousands of IDPs will return to their communities of origin, likely to locations in need of post-conflict reconstruction and with severely AR-RAQQA limited basic services. Additionally, significant challenges are arising as displacement flows into JURNEYYEH Ar-Raqqa from Deir-ez-Zor governorate increase with a corresponding escalation of conflict. "Ar-Raqqa KARAMA Kms 0 5 10 20 30 40 • It is estimated over 300,000 persons were displaced within and from Ar-Raqqa governorate TURKEY 2 AL-THAWRAH between November 2016 and September 2017. Although many displacements were temporary or AR-RAQQA MAADAN within the governorate, a portion of IDPs have left Ar-Raqqa governorate, primarily to Aleppo or Al- Areas of control SABKA Hasakeh governorates, followed by Damascus, with smaller numbers leaving Syria entirely. Generally, MANSURA as conflict progressed, IDPs fled to territory that came under control of the Syrian Democratic Forces SDF (SDF) in previous phases of fighting, rather than further into ISIL-held territory. -

Syria Cvdpv2 Outbreak Situation Report # 9 15 August 2017
Syria cVDPV2 outbreak Situation Report # 9 15 August 2017 cVDPV2 cases in Deir Ez-Zor, Raqqa and Homs governorates, Syria, 2017 Summary New cVDPV2 cases this week: 3 Total number of cVDPV2 cases: 33 Outbreak grade: 3 Index case Location: Mayadeen district, Deir Ez-Zor gover- norate Onset of paralysis: 3 March 2017 Age: 22 months Vaccination status: 2 OPV doses/zero IPV Most recent cases Tadmour district, Homs governorate Onset of paralysis: 10 July 2017, age: 24 months, vaccination status: 1 OPV/zero IPV Deir Ez-Zor district, Deir Ez-Zor governorate Onset of paralysis: 30 June 2017, age: 27 months, vaccination status: 5 OPV/1 IPV Boukamal district, Deir Ez-Zor governorate Onset of paralysis: 25 June 2017, age: 16 months, vaccination status: 1 OPV/2 IPV The boundaries and names shown and the designations used on this map do not imply official endorsement or Affected districts acceptance by the United Nations. Source: Syrian Arab Republic, Administrative map, DFS, United Nations 2012 Mayadeen, Deir Ez-Zor, Boukamal (Deir Ez-Zor); Key highlights Tell Abyad (Raqqa); Tadmour (Homs) Immunization response Three (3) new cases of cVDPV2 were reported this week from three new districts: Two rounds each planned for Deir Ez-Zor and 1 case from Deir Ez-Zor district, Deir Ez-Zor governorate; 1 from Boukamal district, Raqqa governorates. First round in Raqqa started Deir Ez-Zor governorate; and 1 from Tadmour district, Homs governorate. 12 August. First round in Deir Ez-Zor held 22-26 July. Second round scheduled for 19-23 August. Five districts in three governorates are now infected. -

Regional Quarterly Update - June 2015 Basic Needs 3
STRATEGICSITUATION OBJECTIVE:OVERVIEW: REGIONAL QUARTERLY UPDATE - JUNE 2015 BASIC NEEDS 3 Core relief items continue to meet the most PARTNERS JOINING FORCES TO HELP THOSE MOST IN NEED IN JORDAN urgent needs of newly arrived Syrian refugees UNHCR and UNICEF are working in partnership to provide assistance that directly targets Jan-31 in Iraq and Turkey some of Jordan’s most vulnerable refugee children in a salient example of 3RP partners 2441507 joining their expertise to assist those most in need in Jordan. The UNICEF child cash grant programme started in February 2015 and is expected to REGIONAL HIGHLIGHTS: benefit, over a six month period, over 54,000 children from 16,500 vulnerable Syrian families living outside of camps. These families are part of the 30,000 most vulnerable With June marked by an influx of refugees to Turkey and heavy heat in Iraq, the households identified as particularly vulnerable by the Inter-agency Vulnerability provision of core relief items (CRIs) remained key to help meet the immediate needs Assessment Framework (VAF). The most vulnerable families include those with children of refugees. living below the poverty line, female headed households, households with children under Intense fighting in Tell Abyad district in Ar-Raqqa Governorate, Syria, led over 21,000 UNHCR/Sebastian Rich five years, and families taking care of unaccompanied and separated children. Each child Syrian refugees crossing the Ackakale border into Turkey, according to Turkish Sector Response Summary: is provided with a monthly grant of JOD 20 (approximately USD 28). authorities. The partners of the basic needs sector responded to the needs of the 1,904,095 Refugees & Local UNICEF’s support is channelled through UNHCR’s cash assistance platform, new arrivals through the provision of blankets, pillows, floor mats, mattresses, dignity Community Members targeted for complementing UNHCR’s existing assistance to vulnerable urban refugee households.