Comparative Study of Six Pear Cultivars in Terms of Their Phenolic And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

About Apples & Pears
Today’s program will be recorded and posted on our website and our Facebook page. https://ucanr.edu/sites/Amador_County_MGs/ Look under “Classes & Events” then “Handouts & Presentations” from our home page. Today’s handouts will also be posted here. https://www.facebook.com/UCCEAmadorMG/ Look for “Facebook Live” during the meeting or find the video link on our feed. Have a Gardening Question? UC Master Gardeners of Amador County are working by phone and email to answer your gardening questions! Phone: 209-223-6838 Email: [email protected] Facebook: @UCCEAmadorMG Not in Amador County? Find your local Master Gardener program by doing a web search for “UCCE Master Gardener” and your county name. Your Home Orchard: APPLES & PEARS by UCCE Amador County Master Gardeners John Otto & Hack Severson - October 10, 2020 To be discussed • Home Orchard Introduction • What are Apples & Pears? • History & “Lore” • Orchard Planning • Considerations for Selection • Varieties for the foothills Home Orchard Introduction 8,500’ 4,000’ 3,000’ 2,000’ 1,000’ 300’ Amador County There are a variety of fruit and nut trees grown in the Sierra Foothills but elevations and micro-climates make selection an adventure. Stone Fruits: Almond, Apricot, Cherry, Nectarine, Peach, Plumb, Prunes, Plumcots Nut Crops: Chestnuts, Filberts (Hazelnut), Pecans, Walnut, Almond (truly a stone fruit) Citrus: Lemon Lime, Orange (incl. Mandarin, Tangarine, others), Grapefruit, Kumquat, Tangelo Pome Fruits: Apple; Pear; Pomegranates; Quince “The Home Orchard”, ANR publication #3485 https://anrcatalog.ucanr.edu/Details.aspx?itemNo=3485 Apples and Pears Are?? • How are Apples and Pears different from other fruits?? • They are “Pome” fruit (also pomegranate & quince). -

Tapas Simposio Peras
GROWln ~J. AÁl-Ja ttvlv~,~V'J XIII INTERNATIONAL PEAR SYMPOSIUM monTEUIDEO - URUGUAY 1 1 DECEffiBER 4" - 7" 2018 Municipal Palace, Montevideo, Uruguay www.pear2018.uy Argentina 1 1 Belgium M.C. Dussi Serge Remy Universidad Nacional de Comahue Jeff Vercammen Gabriela Calvo PC Fruits María Dolores Raffo INTA - China ... Germany Yuanwen Teng Michael Blanke Zhejiang University University of Bonn m Portugal Karen 1Theron Cristina Ollvelra Un iversity of Stellen bosch Instituto Superior de Agronomía ® 1 United States of America .:....- spa1n . Amit Dhingra Luis Asin Stefano Musacchi IRTA Washington State University Todd Einhorn Michigan State University ~ Uruguay Rachel Elkins Danilo Cabrera University of California Cooperative Extension Terence Robinson Alicia Castillo Cornell Univesity Carolina Leoni Diego Maesa Valentina Mujica Jorge Soria Roberto Zoppolo INIA Pedro Mondino Vivian Severino Ana Cecilia Silveira Facultad de Agronomía Organizing committee Conveners Financia! administration Roberto Zoppolo Esteban Cisneros Danilo Cabrera Communication Support group Mónica Trujillo Maximiliano Dini Carolina Fasiolo Web Valentina Mujica María Emilia Guinovart Julio Pisano Pablo Rodríguez ~ Contact lnformation Yésica Bernaschina ..WWW Website: www.pear2018.uy '-"" E-mail: [email protected] ISBN: 978-9974-38-421-7 Declarations of interest and sponsorship The XIII lnternational Pear Symposium has been declared as an event of public interest by: UruguayNatura/ mee MINISTERIO DE GANADERÍA AGRICULTURA Y PESCA Ministerio de Turismo r.f.11 Intendencia Sponsored by: im de Montevideo Host country Uruguay is a country in Southern South America, bordering the South Atlantic Ocean, between Argentina and Brazil. With an area of approximately 176,000 km 2, Uruguay is geographically the second-smallest nation in South America, afterSuriname. -

Factors Affecting Shrivelling and Friction Discolouration Of
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Stellenbosch University SUNScholar Repository FACTORS AFFECTING SHRIVELLING AND FRICTION DISCOLOURATION OF PEARS (Pyrus communis L.) BY G.E. BURGER Thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Agriculture at the University of Stellenbosch. South Africa December 2004 SUPERVISOR Dr M. Huysamer – Department of Horticultural Science, University of Stellenbosch. CO-SUPERVISOR Prof G. Jacobs – Department of Horticultural Science, University of Stellenbosch. DECLARATION I, the undersigned, hereby declare that the work contained in this thesis is my own original work and that I have not previously in its entirety or in part submitted it at any university for a degree. _____________________________ ______________________ Signature Date i SUMMARY Shrivelling and friction discolouration (FD), as postharvest disorders, negatively influence the marketability and potential shelf life of pears. By investigating the contributing factors in each of the disorders, the potential involvement of handling and storage variables were determined. This allowed for a better understanding of the responsible factors that create susceptible environments for these disorders to occur. From the moment that pears are harvested they lose weight by means of transpiration and, to a lesser extent, respiration. When excessive losses are experienced, the fruit will appear shrivelled and the marketability and shelf life are negatively influenced. By minimizing the rates of weight loss, the occurrence of shrivelling among pears during the postharvest handling can be lessened. The periods that proved to be most conducive to shrivelling (during a simulated postharvest handling duration) were where temperatures above 0 °C were experienced. -

THE MODERN Modern Cocktails
THE MODERN Modern Cocktails Bourbon Elijah Craig Small Batch, Apple Cider, Spiced Pear, Sherry 18 Rum El Dorado 3 year, Toasted Coconut, Lime, Turmeric 18 Vodka Spring 44, Aquavit, Ginger, Lemon, Honey, Rosemary 18 Pear Brandy Purkhart, Lapsang Tea, Almond, Champagne, Cinnamon 18 Mezcal Del Maguey Vida, Green Chillies, Lime, Aperol 18 Blanco Tequila Cimarrón, Lime, Almond, Bitter Orange 18 Spiced Rum (served hot) Far North Ålander, Brown Butter, Steamed Milk, Cocoa 18 Reposado Tequila Cimarrón, Calvados, 5-Spice, Pomegranate 20 Blanche Armagnac Château de Laubade, Tanqueray No. 10, Verjus 20 Rye High West Double Rye, Cognac, Anise Hyssop, Fernet 20 Gin Barr Hill, Green Chartreuse, Lime, Tonic, White Pepper 20 The Modern is a non-tipping restaurant. Hospitality Included. 1 Zero-Proof Cocktails Classic Cocktails Sherry Cobbler Pear and Thyme Soda Lustau East India Solera, Citrus, Sugar, Mint 16 Bosc Pear, Cider Vinegar, Soda Water, Fresh Thyme 10 Corpse Reviver #2 London Dry Navy Strength Gin, Lemon, Cointreau, Cocchi Americano 18 Tonic, Juniper, Botanicals, Cucumber 10 Airmail 9-5 Aged Rum, Lime, Honey, Champagne 18 Cranberry, Lemon, Ginger Soda 10 Pisco Sour Harvest Bellini Peruvian Pisco, Citrus, Eggwhite, Bitters 18 Sparkling Apple Cider, Pumpkin, Passionfruit, Black Pepper 10 La Louisiane Rye, Sweet Vermouth, Benedictine, Absinthe, Bitters 20 Juices Sparkling Wines Navarro Vineyards Pinot Noir Juice, California 10 Duché de Longueville Sparkling Apple Cider, France 10 Blanc de Noirs Bruno Dangin, Crémant de Bourgogne, Burgundy NV 18 Champagne Sodas 7 Boylan’s Root Beer Blanc de Blancs Fentiman’s Ginger Beer Petit Le Brun et Fils, Avize NV 24 Q Ginger Ale Q Grapefruit Soda Brut Q Tonic Gosset, Grande Réserve, Aÿ NV 28 Brut Rosé Philippe Gonet, Le Mesnil-sur-Oger NV 30 Billecart-Salmon, Mareuil-sur-Aÿ (Half Bottle) NV 110 Grande Cuvée Krug, Reims (Half Bottle) NV 120 Rosé Wine Cinsault Blend C h â t e a u l e s Va l e n t í n e s , C ô t e s d e P r o v e n c e 2 0 1 5 1 8 The Modern is a non-tipping restaurant. -

Summary of the 2002 Pacific Northwest of USA Pear Rootstock Trials: Performance of 'D'anjou' and 'Golden Russet Bosc' Pear on Eight Pyrus Rootstocks Todd C
80 JOURNAL OF THE AMERICAN POMOLOGICAL SOCIETY Journal of the American Pomological Society 67(2): 80-88 2013 Summary of the 2002 Pacific Northwest of USA Pear Rootstock Trials: Performance of 'd'Anjou' and 'Golden Russet Bosc' Pear on Eight Pyrus Rootstocks TODD C. EINHORN1,4, STEVE CASTAGNOLI1, TIMOTHY J. SMITH2, JANET TURNER1, AND EUGENE MEILKE3 Additional index words: yield, yield efficiency, tree size, fruit size, suckering, precocity, tree survival Abstract In 2002, a multi-site pear rootstock trial was established to evaluate ‘d’Anjou’ and ‘Golden Russet Bosc’ pear (Pyrus communis L.) performance on eight Pyrus rootstocks in the Pacific northwestern US states of Oregon and Washington. The plantings were conducted under the multi-state cooperative research project NC-140. Root- stock genotypes tested at all three sites included, Fox 11 (Bologna, Italy), two clonal Old Home × Farmingdale selections (OH×F 87 and OH×F 40; US), 708-36 (East Malling, UK), and Pyrodwarf and Pyro 2-33 (Geisenheim, Germany). In addition, Fox 16 (Bologna, Italy) was evaluated at the two WA sites, and Winter Nelis seedling was evaluated at the OR site. Summarized across all sites, OH×F 87 had the highest yields and yield efficiency (YE) and the largest average fruit size; Fox 11 and 708-36 produced the smallest trees; and Pyrodwarf produced significantly low cumulative yields of smaller fruit, possessed the lowest cumulative YE, and suckered profusely (counted in OR, and visually observed in WA). In general, ‘d’Anjou’ trees were larger than ‘Golden Russet Bosc’ for a given rootstock. For the inherently vigorous cultivar ‘d’Anjou’, 708-36 produced the smallest trees followed by the Fox selections, but none of these genotypes had high YE. -

Fire Blight Control Program in Organic Fruit
The Organic Center | www.organic-center.org Critical Issue Report: Fire Blight Control Program in Organic Fruit Grower Lessons and Emerging Research for Developing an Integrated Non-Antibiotic Fire Blight Control Program in Organic Fruit Harold Ostenson* and David Granatstein* November 2013 The Organic Center Fire Blight Control Program in Organic Fruit November 2013 2 Table of Contents Letter from The Organic Center . 3 Important Note to Readers . 4 1. Introduction and Purpose . 5 2. Management in Transitioning to Non-Antibiotic Fire Blight Control . 6 3. Integrated Systems Approach to Non-Antibiotic Control of Fire Blight in Apples . 8 4. Integrated Systems Approach to Non-Antibiotic Control of Fire Blight in Pears . 11 5. Emerging Research and Control Options . 14 Notes . 16 References and Further Reading . 18 Photos . 19 Appendix 1. Selecting Cultivars with Greater Fire Blight Resistance Fire blight resistance . 22 Listings of resistant cultivars . 23 Literature Cited . 28 * Harold Ostenson, tree fruit consultant, Wenatchee, WA ** David Granatstein, extension educator, Wenatchee, WA The Organic Center Fire Blight Control Program in Organic Fruit November 2013 3 The Organic Center is pleased to present this publication, looking at lessons learned on organic methods for controlling the spread of fire blight without the use of antibiotics. Fire blight is a serious problem for apple and pear growers in the US; it is highly infectious and can kill entire trees. With growers now spending up to $20,000 per acre to establish an orchard, the risk of severe tree injury or loss from fire blight needs to be controlled. In the past, the antibiotics streptomycin and oxytetracycline have been the key fire blight con- trols used by most organic growers. -

Produce Talk
Produce Talk Volume 29 Issue 33 August 23, 2018 Item of the Week: PEARS Sweet, delicious and rich flavored pears offer crunchiness of apples yet juicy as peach and nectarine. They are widely popular, particularly in the whole of the northern hemisphere, for their unique nutrient qualities. Most North American pears are grown in Oregon and Washington, and the harvest months listed here reflect that. You can find some variety of pear in season in North America from August through May (and even into June some years). Externally, its skin is very thin; and depending upon the cultivar type, it can be green, red-orange or yellow-orange in color. Inside, its off-white color flesh is soft and juicy. However, in the case of completely ripe fruits, its flesh may turn to grainy texture with gritty sensation while cutting with a knife. The center of the fruit is more or less similar to an apple in appearance with centrally located tiny inedible seeds. Health Benefits of Pears Pears fruit is packed with health benefiting nutrients such as dietary fiber, antioxidants, minerals, and vitamins, which are necessary for optimum health. Total measured antioxidant strength (ORAC value) in pears is 2941 µmol TE/100 g. Pears are a good source of dietary fiber. 100 g fruit provides 3.1 g or 8% of fiber per 100 g. Regular eating of this fruit may offer protection against colon cancer. Most of the fiber in them is a non-soluble polysaccharide (NSP), which functions as a good bulk laxative in the gut. Additionally, its gritty fiber content binds to cancer- causing toxins and chemicals in the colon, protecting its mucous membrane from contact with these compounds. -

"Register of New Fruit and Nut Varieties"
Register of New Fruit and Nut Varieties Brooks and Olmo List 35 Edited by James N. Cummins1 Department of Horticultural Sciences, New York State Agricultural Experiment Station, Cornell University, Geneva, NY 14456 ADDENDA AND REVISIONS Redglobe.-Described in List 32. Plant pat. 4787, 10 Nov. 1981. Symphony. -Described in List32. Plantpat. 5013, 29 Mar. 1983. APPLE Freedom. -Described in List 34. Plant pat. 5723, 22 Apr. 1986. NECTARINE Jonalicious (Daniel). -Synonym added; plant pat. 1777, 9 Dec. 1958. Stark GulfPride (Zaipride). -Described in List 34. Plant pat. Nured® Jonathan (Improved Red Jonathan) -Synonym added; 5461, 7 May 1985. plant pat. 2650, 5 July 1966. Stark HoneyGlo (Anderhone). -Described in List 32. Plant pat. Paulared (Summer Mac). -Synonym added; plant pat. 2800, 12 4789, 10 Nov. 1981. Mar. 1968. Stark® Blushing Golden™ (Griffith Gold) -Synonym added; plant pat. 2835, 1 Oct. 1968. PEACH Starkspur Compact Red Delicious (Cascade Compact Red Delicious).-Described in List 32. Plant pat. 4811, 26 Jan. 1982. Eldorado. -Described in List 32. Plant pat. 4780, 20 Oct. 1981. Starkspur Dixired Delicious (Hared). -Described in List 32. Plant Stark® Finale. Described in List34. Plant pat. 5655, 4 Feb. 1986. pat. 5547, 3 Sept. 1985. Stark® Gulf Queen (Zaiqueen). -Described in List34. Plantpat. Starkspur Law Rome (PeaceValley) -Described in List 32. Plant 5463, 7 May 1985. pat. 4793. 24 Nov. 1981. Starkspur UltraStripe Delicious (Jenred) -Described in List 32. Plant pat. 5472, 21 May 1985. RASPBERRY Starkspur Winter Banana (Frecon). -Described in List33. Plant pat. 4901, 26 Oct. 1982. Royalty. Described in List 33. Plant pat. 5405, 12 Feb. -
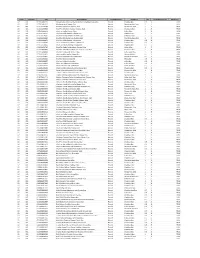
Catid Subcatid UPC Productname Unitofmeasure Shortdesc Qty Unitofmeasureid Wildcard?
CatID SubCatID UPC ProductName UnitOfMeasure ShortDesc Qty UnitOfMeasureID Wildcard? 02 001 011110586919 Private Selection Extra Sharp Vermont Cheddar Cheese 8oz Pound Cheddar 8oz 0.5 6 TRUE 02 001 011110596031 Monterey Jack Cheese 16oz Pound Monterey Jack 16oz 1 6 TRUE 02 001 011161460497 Shurfine Mozzarella Cheese 16oz Pound Mozzarella 16oz 1 6 TRUE 02 001 011161460534 ShurFine Medium Cheddar Cheese 16oz Pound Cheddar 16oz 1 6 TRUE 02 001 011161460602 ShurFine Colby Cheese 16oz Pound Colby 16oz 1 6 TRUE 02 001 011161460619 Shurfine Mild Cheddar Cheese 16oz Pound Cheddar 16oz 1 6 TRUE 02 001 011161460626 Shurfine Sharp Cheddar Cheese 16oz Pound Cheddar 16oz 1 6 TRUE 02 001 011161460640 ShurFine Monterey Jack Cheese 16oz Pound Monterey Jack 16oz 1 6 TRUE 02 001 011161460879 Shurfine Mild Cheddar Cheese 8oz Pound Cheddar 8oz 0.5 6 TRUE 02 001 011161460886 ShurFine Medium Cheddar Cheese 8oz Pound Cheddar 8oz 0.5 6 TRUE 02 001 011161460893 ShurFine Sharp Cheddar Cheese 8oz Pound Cheddar 8oz 0.5 6 TRUE 02 001 011161461746 ShurFine Colby Midget Horn Cheese 16oz Pound Colby 16oz 1 6 TRUE 02 001 011161461760 ShurFine Mild Midget Horn Cheddar Cheese 16oz Pound Cheddar 16oz 1 6 TRUE 02 001 011161466468 Shurfine Colby Jack Cheese 16oz Pound Colby Jack 16oz 1 6 TRUE 02 001 011161466819 ShurFine Mozzarella Cheese 8oz Pound Mozzarella 8oz 0.5 6 TRUE 02 001 011161466826 Shurfine Monterey Jack Cheese 8oz Pound Monterey Jack 8oz 0.5 6 TRUE 02 001 011161466840 Shurfine Swiss Cheese 8oz Pound Swiss 8oz 0.5 6 TRUE 02 001 011161466857 Shurfine Colby Cheese -

Response of Bosc and Cold Snap™ Pears to Thinning with NAA, 6-BA, ACC and S-ABA
Canadian Journal of Plant Science Response of Bosc and Cold Snap™ pears to thinning with NAA, 6-BA, ACC and S-ABA Journal: Canadian Journal of Plant Science ManuscriptFor ID CJPS-2017-0258.R2 Review Only Manuscript Type: Article Date Submitted by the Author: 31-Oct-2017 Complete List of Authors: Cline, John; University of Guelph Ontario Agricultural College, Plant Agriculture Carter, Kathryn; Ontario Ministry of Agriculture, Food and Rural Affairs Gunter, Amanda; University of Guelph, Plant Agriculture Bakker, Catherine; University of Guelph, Plant Agriculture Green, Amanda; Ontario Ministry of Agriculture Food and Rural Affairs Keywords: Pyrus, chemical thinning, crop load management, plant bioregulators https://mc.manuscriptcentral.com/cjps-pubs Page 1 of 39 Canadian Journal of Plant Science Response of Bosc and Cold Snap™ pears to thinning with NAA, 6-BA, ACC and S-ABA. J.A. Cline1*, K. Carter2 A. Gunter3, C. Bakker3, and A.C. Green4 1 Associate Professor, Tree Fruit Physiology, Department of Plant Agriculture, Ontario Agricultural College, University of Guelph, 1283 Blueline Rd, Simcoe, ON N3Y 4N5 Email: [email protected] 2Tender Fruit and Grape Production Specialist, Ontario Ministry of Agriculture, Food and Rural Affairs, 4890 Victoria Ave N, Vineland Station, ON, L0R 2E0 3 Research Technician, Physiology, Department of Plant Agriculture, Ontario Agricultural College, University of Guelph, 1283 Blueline Rd, Simcoe, ON N3Y 4N5 4Tree Fruit Specialist, ForOntario ReviewMinistry of Agriculture, Only Food and Rural Affairs, 1283 Blueline Rd, Simcoe, ON N3Y 4N5 * Corresponding author Email:[email protected] ABSTRACT Adjusting the crop-load of European pears [Pyrus communis L.] by hand thinning is currently required to ensure marketable size of most cultivars grown in Ontario. -
Composition and Storage of Pear Cultivars from Nagykanizsa
International Journal of Horticultural Science 2011, 17 (1 –2): 63 –68. Agroinform Publishing House, Budapest, Printed in Hungary ISSN 1585-0404 Composition and storage of pear cultivars from Nagykanizsa Tóth-Markus, M. 1, Bánáti, D. 1, Adányi, N. 1, Boross, F. 1, Konrád-Németh, C. 2, Szabó, Z., 3 Soltész, M. 4 & Nyéki, J. 3 1Central Food Research Institute, Hungary, H-1022 Budapest, Herman Ottó Street 15 tel.: +36 1 3558838 e-mail: [email protected] 2Gyümölcskert cPlc Hungary, H-8900 Nagykanizsa, Csengery street 90 3University of Debrecen Centre for Agricultural and Applied Economic Sciences, H-4032 Debrecen, Böszörményi út 138., Hungary 4Collage of Kecskemét, Faculty of Horticulture, H-6000 Kecskemét, Erdei Ferenc tér 1 –3. Summary: The composition of five pear varieties (‘Abate Fètel’, ‘Bosc’, ‘Williams’, ‘Conference’, ‘Packham’s Triumph’) grown in Nagy- kanizsa was investigated in three consecutive years (2008, 2009, 2010). A storage experiment was performed in 2008. Four winter pear cultivars were kept in an ULO store for four months and their parameters measured after two and four months. The parameters tested were: size, weight, water soluble solids, titratable acidity, glucose, fructose, sucrose, water soluble pectin, total polyphenols, free radical scavenging capacity, copper and zinc content. The fruits of ‘Conference’ and ‘Bosc’ varieties were found to contain the highest sucrose and total sugar content, while ‘Abate Fétel’ had the lowest sucrose and highest glucose levels among cultivars tested. ‘Williams’ pear was the most acidic. Brix, total sugar, sucrose and water soluble pectin were decreased during storage. Titratable acidity slightly decreased in fruits of Conference pear. -

Phenolic Compounds and Antioxidant Capacity of Pear As Affected by Rootstock and Cultivar
MITTEILUNGEN KLOSTERNEUBURG 70 (2020): 308-319 MILOŠEVIĆ et al. PHENOLIC COMPOUNDS AND ANTIOXIDANT CAPACITY OF PEAR AS AFFECTED BY ROOTSTOCK AND CULTIVAR Tomo Milošević1, Nebojša Milošević2 and Pavle Mašković3 1 Department of Fruit Growing and Viticulture, Faculty of Agronomy, University of Kragujevac RS- 32000 Čačak, Cara Dušana 34 2 Department of Pomology and Fruit Breeding, Fruit Research Institute Čačak RS-32000 Čačak, Kralja Petra I/9 3 Department of Chemistry and Chemical Engineering, Faculty of Agronomy, University of Kragujevac RS-32000 Čačak, Cara Dušana 34 E-Mail: [email protected] During two consecutive years we investigated the influence of two traditional quince rootstocks and three commer- cial pear cultivars on the phenolic compounds content and the antioxidant capacity in whole fruit. Results showed that the impact of rootstocks was lesser than the impact of cultivars. Quince BA.29 showed the higher vitamin C content, total antioxidant capacity, condensed tannins and gallotannins, whereas quince MA induced better values of inhibitory activity against lipid peroxidation and metal chelating activity. 'Abbé Fétel' on both rootstocks was the cultivar with the highest vitamin C and total anthocyanin content (TAc), total phenolic (TPC) and total flavonoids contens (TFC), total antioxidant capacity (TAC), condensed tannins and gallotannins in comparison with 'Starking Delicious' and 'Beurré Bosc'. Values of DPPH scavenging activity, inhibitory activity against lipid peroxidation, me- tal chelating activity and hydroxyl radical scavenging activity were statistically similar and higher in both ‘Starking Delicious’ and ‘Beurré Bosc’ in relation to ‘Abbé Fétel’, i. e. they had a lower antioxidant capacity than 'Abbé Fétel'. Keywords: anthocyanins, bioactive compounds, DPPH scavenging activity, fruit, Pyrus communis L., vitamin C Einfluss von Unterlage und Sorte auf Phenolgehalte und antioxidative Kapazität von Birnen.