The Evolution of White Blood Cell Differential Technologies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mature and Immature/Activated Cells Fractionation: Time for a Paradigm Shift in Differential Leucocyte Count Reporting?
diagnostics Article Mature and Immature/Activated Cells Fractionation: Time for a Paradigm Shift in Differential Leucocyte Count Reporting? Rana Zeeshan Haider 1,2,* , Najeed Ahmed Khan 3, Eloisa Urrechaga 4 and Tahir Sultan Shamsi 2 1 Baqai Institute of Hematology, Baqai Medical University, Karachi 75340, Pakistan 2 National Institute of Blood Disease (NIBD), Karachi 75300, Pakistan; [email protected] 3 Department of Computer Science, NED University of Engineering and Technology, Karachi 75270, Pakistan; [email protected] 4 Core Laboratory, Galdakao-Usansolo Hospital, 48960 Galdakao, Spain; [email protected] * Correspondence: [email protected]; Tel.: +92-343-507-1271 Abstract: Leucocytes, especially neutrophils featuring pro- and anti-cancerous characteristics, are involved in nearly every stage of tumorigenesis. Phenotypic and functional differences among mature and immature neutrophil fractions are well reported, and their correlation with tumor progression and therapy has emerging implications in modern oncology practices. Technological advancements enabled modern hematology analyzers to generate extended information (research parameters) during complete blood cell count (CBC) analysis. We hypothesized that neutrophil and lymphocyte fractions-related extended differential leucocytes count (DLC) parameters hold superior diagnostic utility over routine modalities. The present study was carried out over a four-and-a-half-year period wherein extended neutrophil (immature granulocyte [IG] and mature neutrophil [NEUT#&]), and lymphocyte (activated/high fluorescence lymphocyte count [HFLC] and resting lymphocyte Citation: Haider, R.Z.; Khan, N.A.; [LYMP#&]) parameters were challenged over routine neutrophil [NEUT#] and lymphocyte [LYMP#] Urrechaga, E.; Shamsi, T.S. Mature and Immature/Activated Cells items in a study population of 1067 hematological neoplasm patients. -

Seed Haematology
SYSMEX EDUCATIONAL ENHANCEMENT AND DEVELOPMENT | APRIL 2016 SEED HAEMATOLOGY Challenges in monocyte counting Introduction Morphologically, monocytes are the largest leukocytes, with Monocytes are a type of leukocytes (white blood cells). They sizes varying between 10 and 20 µm. They usually have a perform an important part of the immune defence of the large nucleus and a moderate amount of cytoplasm, which is organism. One of their functions is to destroy bacteria by grey-blue after May-Grünwald Giemsa staining and may phagocytosis and that is the reason why vacuoles are often contain fine, evenly distributed granules and sometimes seen in the cytoplasm of these cells. vacuoles (Figs. 1 and 2). The reference ranges for monocytes are 5.2 – 15.2 % or 0.29 – 0.95 x 109/L for men and 4.2 – 11.8 % Monocytes are produced in the bone marrow from precur- or 0.25 – 0.84 x 109/L for women [1]. Values outside this sor cells called monoblasts, which themselves are derived range do not necessarily indicate disease. It is recom- from haematopoietic stem cells. Monocytes circulate in the mended to always examine reference ranges for suitability bloodstream for about one to three days and then typically in a given patient population according to the method enter tissue throughout the body. In tissue, monocytes recommended by the International Federation of Clinical mature into different types of macrophages in different ana- Chemistry and Laboratory Medicine [2]. tomical locations. Macrophages are responsible for protect- ing tissue from foreign substances (microbes, cancer cells, cellular debris) in a process called phagocytosis. -

IDEXX Procyte Dx* Hematology Analyzer Operator's Guide
IDEXX ProCyte Dx* Hematology Analyzer Operator’s Guide Proprietary Rights Notice Information in this document is subject to change without notice. Companies, names and data used in examples are fictitious unless otherwise noted. No part of this document may be reproduced or transmitted in any form or by any means, electronic, mechanical or otherwise, for any purpose, without the express written permission of IDEXX Laboratories. IDEXX Laboratories may have patents or pending patent applications, trademarks, copyrights or other intellectual or industrial property rights covering this document or subject matter in this document. The furnishing of this document does not give a license to these property rights except as expressly provided in any written license agreement from IDEXX Laboratories. © 2014 IDEXX Laboratories, Inc. All rights reserved. • 06-0002359-00 *ProCyte Dx, Laminar Flow Impedance, Smart Flags, IDEXX VetLab, IDEXX SmartLink, IDEXX InterLink, Catalyst, Catalyst Dx, IDEXX UA, IDEXX VetStat, VetTest, VetLyte, LaserCyte, Coag Dx, VetCollect, IDEXX VetTrol, SNAP, SNAP cPL, and 4Dx are trademarks or registered trademarks of IDEXX Laboratories, Inc. or its affiliates in the United States and/or other countries. e-CHECK is a trademark or registered trademark of Sysmex Corporation. Vacutainer and Hemoguard are trademarks of Becton, Dickinson and Company. VetAutoread is a trademark of QBC Diagnostics, Inc. Linksys and EtherFast are registered trademarks of Cisco Systems, Inc. All other product and company names and logos are trademarks -

Hematology and Clinical Chemistry Reference Ranges for Laboratory-Bred Natal Multimammate Mice (Mastomys Natalensis)
viruses Article Hematology and Clinical Chemistry Reference Ranges for Laboratory-Bred Natal Multimammate Mice (Mastomys natalensis) David M. Wozniak 1 , Norman Kirchoff 1, Katharina Hansen-Kant 1, Nafomon Sogoba 2, David Safronetz 3,4 and Joseph Prescott 1,* 1 ZBS5—Biosafety Level-4 Laboratory, Robert Koch-Institute, 13353 Berlin, Germany; [email protected] (D.M.W.); [email protected] (N.K.); [email protected] (K.H.-K.) 2 International Center for Excellence in Research, Malaria Research and Training Center, Faculty of Medicine, Pharmacy and Dentistry, University of Sciences, Techniques and Technologies of Bamako, Bamako 91094, Mali; [email protected] 3 Laboratory of Virology, Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840, USA; [email protected] 4 Zoonotic Diseases and Special Pathogens, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, MB R3E 3M4, Canada * Correspondence: [email protected] Abstract: Laboratory-controlled physiological data for the multimammate rat (Mastomys natalensis) are scarce, despite this species being a known reservoir and vector for zoonotic viruses, including the highly pathogenic Lassa virus, as well as other arenaviruses and many species of bacteria. For this reason, M. natalensis is an important rodent for the study of host-virus interactions within laboratory settings. Herein, we provide basic blood parameters for age- and sex-distributed animals in regards to blood counts, cell phenotypes and serum chemistry of a specific-pathogen-monitored M. natalensis Citation: Wozniak, D.M.; Kirchoff, breeding colony, to facilitate scientific insight into this important and widespread rodent species. N.; Hansen-Kant, K.; Sogoba, N.; Safronetz, D.; Prescott, J. -

What Your Hematology Analyzer Can't Tell
Applied Cytology HEMATOLOGY Peer Reviewed Case 1: Lameness & Pyrexia What Your Hematology Analyzer Can’t Tell You Heather L. Wamsley, DVM, PhD, Diplomate ACVP (Clinical Pathology) A 10-month-old, female bloodhound presented for acute onset University of Florida shifting leg lameness and pyrexia (104° F/40° C). Table 1 (page 32) provides blood analysis results; Figures 1 through 3 show the blood films. ollection of a peripheral blood sample allows a practi - tioner the opportunity to perform a complete blood Ccount (CBC), which includes automated analysis of ASK YOURSELF… the blood and microscopic evaluation of the peripheral G Is the patient truly thrombocytopenic as blood smear. suggested by the automated CBC? G Is the cause of clinical signs evident in The peripheral blood smear (blood film) not only confirms the blood film? and refines numeric data reported by the hematology ana - lyzer, but also adds useful information when evaluated by a Intragranulocytic, dark trained microscopist: blue, stippled, irregularly G Red blood cell (RBC) quantity (RBC count, hematocrit, 1 shaped morula adjacent to segmented neutrophil hemoglobin) nucleus; platelets are not G RBC indices (mean cell volume, mean cell hemoglobin visible. ( Wright-Giemsa , concentration, RBC distribution width) 100×) G White blood cell (WBC) count G WBC differential G Platelet count G Platelet indices (mean platelet volume) 2 Each of the following cases highlight different reasons Intragranulocytic, dark blue, stippled, crescent- why it remains important for trained personnel to shaped morula adjacent examine blood films even when an advanced, to the segmented neu - automated hematology analyzer is used to trophil nucleus. ( Wright- Giemsa , 100×) obtain data. -

Complete Blood Count with 5-Part Differential
Laboratory Procedure Manual Analyte: Complete Blood Count Matrix: Whole Blood Method: Complete Blood Count with 5-Part Differential Method No.: Revised: as performed by: Contact: Important Information for Users CDC periodically refines these laboratory methods. It is the responsibility of the user to contact the person listed on the title page of each write-up before using the analytical method to find out whether any changes have been made and what revisions, if any, have been incorporated. Complete Blood Count with 5 Part Differential in Whole Blood NHANES 2013-2014 Public Release Data Set Information This document details the Lab Protocol for testing the items listed in the following table: Lab Number Analyte SAS Label LBXWBCSI White blood cell count (1000 cells/uL) LBXLYPCT Lymphocyte (%) LBXMOPCT Monocyte (%) LBXNEPCT Segmented neutrophils (%) LBXEOPCT Eosinophils (%) LBXBAPCT Basophils (%) LBDLYMNO Lymphocyte number (1000 cells/uL) LBDMONO Monocyte number (1000 cells/uL) Segmented neutrophils number (1000 LBDNENO cells/uL) CBC_H LBDEONO Eosinophils number (1000 cells/uL) LBDBANO Basophils number (1000 cells/uL) LBXRBCSI Red cell count (million cells/uL) LBXHGB Hemoglobin (g/dL) LBXHCT Hematocrit (%) LBXMCVSI Mean cell volume (fL) LBXMCHSI Mean cell hemoglobin (pg) LBXMC MCHC (g/dL) LBXRDW Red cell distribution width (%) LBXPLTSI Platelet count ((1000 cells/uL)) LBXMPSI Mean platelet volume (fL) Complete Blood Count with 5 Part Differential in Whole Blood NHANES 2013-2014 A complete blood count (CBC) is performed in duplicate on all study participants (SPs) aged 1 year and older. The CBC is performed on the Coulter® DxH 800 analyzer using the SP’s EDTA blood tubes which are obtained via the phlebotomy component. -

Bc-5300/5380
BC-5300/5380 深圳:(0755) 81886619 成都:(028) 85322230 南京:(025) 86618592 郑州:(0371) 69316601 青岛:(0532) 80996585 北京:(010) 65810066 哈尔滨:(0451) 53622438 广州:(020) 38107486 重庆:(023) 68603650 合肥:(0551) 5609671 武汉:(027) 85714714 呼和浩特: (0471) 6665621 石家庄:(0311) 86666326 沈阳:(024) 22790288 福州:(0591) 87878859 贵阳:(0851) 5280593 杭州:(0571) 86772633 长沙:(0731) 85178277 乌鲁木齐:(0991) 2837326 天津:(022) 23201536 长春:(0431) 88987811 南宁:(0771) 5501579 昆明:(0871) 31711101 上海:(021) 34619081 南昌:(0791) 6818819 西安:(029) 88360648 太原:(0351) 8308836 大连:(0411) 82529862 海口: (0898) 68575395 苏州:(0512) 69367190 兰州:(0931) 8115539 济南:(0531) 81663350 2013 深圳迈瑞生物医疗电子股份有限公司版权所有。 是深圳迈瑞生物医疗电子股份有限公司的注册商标 国食药监械(准)字2010第3300644号 深圳市南山区高新技术产业园区科技南十二路迈瑞大厦 产品规格如有变化,恕不另行通知,谨以最新技术资料及检验结果为准。 电话: 755 8188 8998 传真: 755 2658 2680 P/N: ENG-CCS-5300/5380-210145x36-20110804 www.mindray.com 美国纽交所股票代码MR BC-5300/5380 Auto Hematology Analyzer Full automatic 5-part differentiation of WBC, 27 parameters, 3 histograms and 1 scattergram Laser scatter, Advanced flow cytometry, Chemical dye method Independent Basophil channel Up to 60 samples per hour 2 Sampling modes: Autoloader and Closed tube Large storage capacity: 40,000 results with graphs Preface to Clinical Case Study for spread product portfolio and an established presence in over 165 Mindray Hematology analyzer BC-5300/5380 countries; Mindray takes the task of supporting current healthcare Dr. Vijay Parekh, Scientific Director in Mindray demands seriously. Microscope and Romanowsky dyes availability have resulted in Clinical case study for BC-5300/5380 hematology analyzer is an accumulation of a vast pool of knowledge about cytological chang- example of that effort. It is a compilation of BC-5300/5380 hemato- es seen in various blood disorders. -

Dxh 500 SERIES HEMATOLOGY ANALYZER
TECHNOLOGY AND CASE STUDIES DxH 500 SERIES HEMATOLOGY ANALYZER TABLE OF CONTENTS DXH 500 SERIES SYSTEM TECHNOLOGY 4 HISTOGRAMS AND DIFF PLOTS 12 DXH 500 SERIES SYSTEM FLAGGING OVERVIEW 14 CASE 1 | NORMAL 20 CASE 2 | SICKLE CELL ANEMIA 22 CASE 3 | MICROCYTIC ANEMIA WITH THROMBOCYTOSIS 24 CASE 4 | ACUTE MYELOID LEUKEMIA WITH THROMBOCYTOPENIA 26 CASE 5 | THROMBOCYTOSIS 28 CASE 6 | AGGREGATED PLATELETS 30 CASE 7 | MICROCYTIC AND HYPOCHROMIC RBC’S 32 CASE 8 | LEUKOCYTOSIS WITH THROMBOCYTOSIS 34 CASE 9 | ACUTE MONOCYTIC LEUKEMIA 36 CASE 10 | LEUKOCYTOSIS WITH IMMATURE GRANULOCYTES 38 CASE 11 | PLASMACELLULAR DYSCRASIA 40 CASE 12 | EOSINOPHILIA 42 DxH 500 SERIES HEMATOLOGY ANALYZER | TECHNOLOGY AND CASE STUDIES 3 DxH 500 SERIES SYSTEM TECHNOLOGY The DxH 500 Series System technology for Hematology incorporates the Coulter Principle and Flow cytometric optical measurement to provide an effective and robust technology for cellular analysis. The Coulter Principle1 The Coulter Principle is an electronic method for counting and sizing particles. Although the Coulter Principle can be used to calculate and size just about any particle, the specific application of this principle in hematology is to count and size White Blood Cells (WBC), Red Blood Cells (RBC), and Platelets (PLT). ELECTRONIC COUNTING AND SIZING BASICS The Coulter Principle (impedance) is used to count and size cells by detecting and measuring changes in electrical resistance when a particle (such as a cell) is suspended in a conductive liquid and passes through a small aperture. As each cell goes through the aperture, it acts as an insulator and momentarily increases the resistance of the electrical path between the submerged electrodes on either side of the aperture (Figure 1). -

Leukocyte Related Parameters in Older Adults with Metabolically Healthy
www.nature.com/scientificreports OPEN Leukocyte related parameters in older adults with metabolically healthy and unhealthy overweight or obesity Shan‑Shan Zhang1,6, Xue‑Jiao Yang1,6, Qing‑Hua Ma2, Yong Xu1, Xing Chen3*, Pei Wang4,5* & Chen‑Wei Pan1* It remains unclear whether leukocyte‑related parameters could be used as biomarkers to diferentiate metabolically unhealthy overweight/obesity (MUO) from metabolically healthy overweight/obesity (MHO). We aimed to examine the diferences in the distribution of leukocyte‑related parameters between older adults with MHO and MUO and the correlations of leukocyte‑related parameters with individual components of metabolic abnormality. In the Weitang Geriatric Diseases Study on older Chinese adults aged 60 years or above, 404 individuals with MHO and 480 with MUO contributed to the analysis. Overweight/obesity was defned as body mass index (BMI) of 25 kg/m2 or more. MHO and MUO were discriminated based on the Adult Treatment Panel III (ATP III) criteria. Leukocyte‑ related parameters were assessed using an automated hematology analyzer. All leukocyte‑related parameters except monocytes were elevated in MUO group compared with MHO group (all P < 0.05). The prevalence of MUO increased by 24% with each 109/L increase of leukocytes after adjusting for confounders in the multiple‑adjusted model (P < 0.01) and each unit elevation of other parameters except lymphocytes and monocytes were signifcantly associated with the presence of MUO (all P < 0.01). Trend tests revealed a linear trend for the association between MUO and all the leukocyte‑ related parameters (all P for trend < 0.05). Signifcant interactions between leukocyte‑related parameters and sex on the presence of MUO were observed (all P value for interaction < 0.05). -

Reliable, Reportable Wbc Differential Results Through Innovative Data Collection and Analysis
DxH 900 Hematology Analyzer RELIABLE, REPORTABLE WBC DIFFERENTIAL RESULTS THROUGH INNOVATIVE DATA COLLECTION AND ANALYSIS VCS 360, DataFusion and enhanced Coulter Principle technology Gain greater confidence in white blood cell (WBC) differentials through innovative data collection and analysis with VCS 360 technology, DataFusion and enhanced Coulter Principle. The DxH 900 hematology analyzer enables laboratories to deliver WBC results with high efficiency and outstanding performance, facilitating abnormal cell detection without the need for additional modes or added cost. The DxH 900 hematology analyzer provides: › Reliable and reportable WBC differential results on the “first run” through VCS 360, DataFusion technology and the enhanced Coulter Principle—without the need for additional modes or reagents. › State-of-the art detection of abnormal cells › Cellular morphology data for disease research in oncology, hematology, infections and anemia Receive reliable and reportable WBC differential results on the “first run” with VCS 360 and DataFusion › DxH 900 WBC differential technology utilizes thorough biophysical characterization of blood cells with five light-scatter angles, digital conductivity and enhanced Coulter Principle. Cells are not altered by stains or other chemical reactions and undergo near-native flow cytometric analysis for correct recognition and classification, even in very abnormal samples › Direct measurement of standard 5-part differential WBC subtypes without relying on calculations to count basophils RELIABLE, REPORTABLE WBC DIFFERENTIAL RESULTS THROUGH INNOVATIVE DATA COLLECTION AND ANALYSIS › WBC flagging, provided by DataFusion technology, enhances analysis and overall efficiency through data cross-referencing from multiple modules—differential, CBC and NRBC analysis › Customizable flagging for disease and patient populations—such as pediatric, oncology and infection disease—supports patient care. -

A Comparison Between the HORIBA Yumizen H500 Point-Of-Care Hematology Analyzer with a 5-Part White Cell Differential and the HORIBA Pentra 120
ORIGINAL ARTICLE A Comparison Between the HORIBA Yumizen H500 Point-of-Care Hematology Analyzer With a 5-Part White Cell Differential and the HORIBA Pentra 120 Timothy Woolley, MSc, CSci, FIBMS, Bethan Jade Davies, MSc, Emma Rutter, MSc, Charlotte Probert, Leanne Fitzgerald, MSc, and William Relf administration of chemotherapeutic drugs. One drawback of POCT Abstract: The release of new and innovative platforms has helped the hematology analyzers however has long been the lack of a 5-part point-of-care testing market to grow and cement its wider uptake in various WBC differential. clinical settings. Driven by the introduction of novel patient pathways often The FBC along with the WBC count is one of the most fre- centered on clinics remote from a laboratory, the need for laboratory grade quently requested laboratory tests. The WBC typically includes parameters and results from point-of-care analyzers is now critical. 5 white cell subgroups and is used to diagnose and monitor a In this paper, we compare the 5-part white blood cell (WBC) differential variety of pathologic conditions such as bacterial and viral infec- from HORIBA Medicals mainline hematology analyzer, the Pentra 120, tions, inflammation, leukemia, immunodeficiency disorders, and and those provided from the recently released Yumizen H500 point-of- postchemotherapy states.2–4 care analyzer. Patients with chemotherapy-induced neutropenia are at risk The Yumizen H500 is based on HORIBA's Micros platform and is aimed at for severe bacterial infections. This risk is often dependent on the point-of-care/primary care market. The Yumizen H500 is capable of the depth and duration of the neutropenia and the type of underly- measuring a full blood count (27 parameters) including a 5-part WBC dif- ing disease, in which case, the use of antibacterial prophylaxis to μ ferential using only 20 L of whole blood in approximately 2 minutes. -
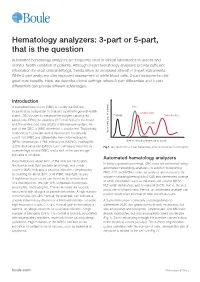
Hematology Analyzers:3-Partor5-Part, Boule.Com the Pulseisrelated Tothecell Size(Fig2)
Hematology analyzers: 3-part or 5-part, that is the question Automated hematology analyzers are frequently used in clinical laboratories to assess and monitor health condition of patients. Although 3-part hematology analyzers provide sufficient information for most clinical settings, trends show an increased interest in 5-part instruments. While 5-part analyzers offer improved assessment of white blood cells, 3-part instruments offer great cost benefits. Here, we describe clinical settings, where 3-part differentials and 5-part differentials can provide different advantages. Introduction A complete blood count (CBC) is usually the first test RBC requested by a physician to evaluate a patient’s general health Lymphocytes status. CBC is used to measure the oxygen-carrying red Platelet Granulocytes blood cells (RBC), the platelets (PLT) that help clot the blood, and the white blood cells (WBC) of the immune system. As MID cells part of the CBC, a WBC differential is conducted. Traditionally, a laboratory technician uses a microscope to manually No. of cells (no. pulses) count 100 WBC and differentiate them into neutrophils (NEU), lymphocytes (LYM), monocytes (MONO), eosinophils Size of cells (fL) (amplitude of pulse) (EOS), and basophils (BASO). Each cell type is reported as Fig 1. Test result from a 3-part hematology analyzer visualized in a histogram. a percentage of total WBC, and a shift in the percentage indicates a condition. Automated hematology analyzers In normal blood, about 60% of the cells are neutrophils. In today’s general screenings, CBC tests are performed using Neutrophils help fight bacteria (and fungi), and a high automated hematology analyzers.