Comparative Efficacy of Linaclotide Versus Other Oral Constipation Treatments in Chronic Constipation: a Network Meta-Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WHO Drug Information Vol. 12, No. 3, 1998
WHO DRUG INFORMATION VOLUME 12 NUMBER 3 • 1998 RECOMMENDED INN LIST 40 INTERNATIONAL NONPROPRIETARY NAMES FOR PHARMACEUTICAL SUBSTANCES WORLD HEALTH ORGANIZATION • GENEVA Volume 12, Number 3, 1998 World Health Organization, Geneva WHO Drug Information Contents Seratrodast and hepatic dysfunction 146 Meloxicam safety similar to other NSAIDs 147 Proxibarbal withdrawn from the market 147 General Policy Issues Cholestin an unapproved drug 147 Vigabatrin and visual defects 147 Starting materials for pharmaceutical products: safety concerns 129 Glycerol contaminated with diethylene glycol 129 ATC/DDD Classification (final) 148 Pharmaceutical excipients: certificates of analysis and vendor qualification 130 ATC/DDD Classification Quality assurance and supply of starting (temporary) 150 materials 132 Implementation of vendor certification 134 Control and safe trade in starting materials Essential Drugs for pharmaceuticals: recommendations 134 WHO Model Formulary: Immunosuppressives, antineoplastics and drugs used in palliative care Reports on Individual Drugs Immunosuppresive drugs 153 Tamoxifen in the prevention and treatment Azathioprine 153 of breast cancer 136 Ciclosporin 154 Selective serotonin re-uptake inhibitors and Cytotoxic drugs 154 withdrawal reactions 136 Asparaginase 157 Triclabendazole and fascioliasis 138 Bleomycin 157 Calcium folinate 157 Chlormethine 158 Current Topics Cisplatin 158 Reverse transcriptase activity in vaccines 140 Cyclophosphamide 158 Consumer protection and herbal remedies 141 Cytarabine 159 Indiscriminate antibiotic -

United States Patent (10) Patent No.: US 8,969,514 B2 Shailubhai (45) Date of Patent: Mar
USOO896.9514B2 (12) United States Patent (10) Patent No.: US 8,969,514 B2 Shailubhai (45) Date of Patent: Mar. 3, 2015 (54) AGONISTS OF GUANYLATECYCLASE 5,879.656 A 3, 1999 Waldman USEFUL FOR THE TREATMENT OF 36; A 6. 3: Watts tal HYPERCHOLESTEROLEMIA, 6,060,037- W - A 5, 2000 Waldmlegand et al. ATHEROSCLEROSIS, CORONARY HEART 6,235,782 B1 5/2001 NEW et al. DISEASE, GALLSTONE, OBESITY AND 7,041,786 B2 * 5/2006 Shailubhai et al. ........... 530.317 OTHER CARDOVASCULAR DISEASES 2002fOO78683 A1 6/2002 Katayama et al. 2002/O12817.6 A1 9/2002 Forssmann et al. (75) Inventor: Kunwar Shailubhai, Audubon, PA (US) 2003,2002/0143015 OO73628 A1 10/20024, 2003 ShaubhaiFryburg et al. 2005, OO16244 A1 1/2005 H 11 (73) Assignee: Synergy Pharmaceuticals, Inc., New 2005, OO32684 A1 2/2005 Syer York, NY (US) 2005/0267.197 A1 12/2005 Berlin 2006, OO86653 A1 4, 2006 St. Germain (*) Notice: Subject to any disclaimer, the term of this 299;s: A. 299; NS et al. patent is extended or adjusted under 35 2008/0137318 A1 6/2008 Rangarajetal.O U.S.C. 154(b) by 742 days. 2008. O151257 A1 6/2008 Yasuda et al. 2012/O196797 A1 8, 2012 Currie et al. (21) Appl. No.: 12/630,654 FOREIGN PATENT DOCUMENTS (22) Filed: Dec. 3, 2009 DE 19744O27 4f1999 (65) Prior Publication Data WO WO-8805306 T 1988 WO WO99,26567 A1 6, 1999 US 2010/O152118A1 Jun. 17, 2010 WO WO-0 125266 A1 4, 2001 WO WO-02062369 A2 8, 2002 Related U.S. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Peptide Therapeutics Designing a Science-Led Strategic Quality Control Program
BioProcess International Peptide SPECIAL REPORT Therapeutics Designing a Science-Led Strategic Quality Control Program INTERTEK PHARMACEUTICAL SERVICES Your partner for regulatory-driven, phase appropriate analytical programs tailored to your molecule. Our experts help you to navigate the challenges of development, regulatory submission, and manufacturing. Peptide Therapeutics Designing a Science-Led Strategic Quality Control Program Shashank Sharma and Hannah Lee ince the emergence of peptide therapeutics in the 1920s with the advent of insulin therapy, the market for this product class has continued to expand with global revenues anticipatedS to surpass US$50 billion by 2024 (1). The growth of peptide therapeutics is attributed not only to improvements in manufacturing, but also to a rise in demand because of an increasingly aging population that is driving an increase in the occurrence of long-term diseases. The need for efficient and low-cost drugs and rising investments in research and development of novel drugs continues to boost market growth and fuel the emergence of generic versions that offer patients access to vital medicines at low costs. North America has been the dominant market for peptide therapeutics, with the Asia–Pacific region Insulin molecular model; the first therapeutic expected to grow at a faster rate. The global peptides use of this peptide hormone was in the market has attracted the attention of key players 1920s to treat diabetic patients. within the pharmaceutical industry, including Teva Pharmaceuticals, Eli Lilly, Novo Nordisk, Pfizer, amino acids to be peptides. Within that set, those Takeda, and Amgen. Those companies have made containing 10 or more are classed as polypeptides. -

(12) Patent Application Publication (10) Pub. No.: US 2011/0158930 A1 Hirata Et Al
US 2011 O15893 OA1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0158930 A1 Hirata et al. (43) Pub. Date: Jun. 30, 2011 (54) METHOD FOR TREATMENT OF RRTABLE Related U.S. Application Data BOWEL, SYNDROME (60) Provisional application No. 61/190.559, filed on Aug. 28, 2008. (75) Inventors: Takuya Hirata, Tokyo (JP); Toshiyuki Funatsu, Tokyo (JP); Publication Classification Yoshihiro Keto, Tokyo (JP); (51) Int. Cl. Shinobu Akuzawa, Tokyo (JP) A63L/78 (2006.01) C07D 403/06 (2006.01) (73) Assignee: ASTELLAS PHARMA INC., A6IPI/00 (2006.01) Tokyo (JP) A6IPL/T2 (2006.01) (52) U.S. Cl. ................................... 424/78.01: 548/302.7 (21) Appl. No.: 13/061,384 (57) ABSTRACT A method for treatment of a patient suffering from irritable (22) PCT Fled: Aug. 27, 2009 bowel syndrome with diarrhea or mixed irritable bowel syn drome, which comprises administering to the patient a thera (86) PCT NO.: PCT/UP2009/064904 peutically effective amount of ramosetron or a pharmaceuti cally acceptable salt thereof in combination with a S371 (c)(1), therapeutically effective amount of polycarbophil or a phar (2), (4) Date: Feb. 28, 2011 maceutically acceptable salt thereof. Patent Application Publication Jun. 30, 2011 Sheet 1 of 2 US 2011/O1589.30 A1 Fig.1 s 120 aw *e 100 aS$ 100 80 isd 80 v s 60 se sk sk 60 40 6 40 se g 20 20 - a k es O -- - - - , , , g- O W 0. 0.3 1 100 300 000 0.3 g/kg 300 mg/kg 0.3 g/kg + s S. -

Linaclotide: a Novel Therapy for Chronic Constipation and Constipation- Predominant Irritable Bowel Syndrome Brian E
Linaclotide: A Novel Therapy for Chronic Constipation and Constipation- Predominant Irritable Bowel Syndrome Brian E. Lacy, PhD, MD, John M. Levenick, MD, and Michael D. Crowell, PhD, FACG Dr. Lacy is Section Chief of Gastroenter- Abstract: Chronic constipation and irritable bowel syndrome ology and Hepatology and Dr. Levenick (IBS) are functional gastrointestinal disorders that significantly is a Gastroenterology Fellow in the affect patients’ quality of life. Chronic constipation and IBS are Division of Gastroenterology and prevalent—12% of the US population meet the diagnostic crite- Hepatology at Dartmouth-Hitchcock Medical Center in Lebanon, New ria for IBS, and 15% meet the criteria for chronic constipation— Hampshire. Dr. Crowell is a Professor and these conditions negatively impact the healthcare system of Medicine in the Division of from an economic perspective. Despite attempts at dietary Gastroenterology and Hepatology at modification, exercise, or use of over-the-counter medications, Mayo Clinic in Scottsdale, Arizona. many patients have persistent symptoms. Alternative treatment options are limited. This article describes linaclotide (Linzess, Address correspondence to: Dr. Brian E. Lacy Ironwood Pharmaceuticals/Forest Pharmaceuticals), a new, first- Division of Gastroenterology and in-class medication for the treatment of chronic constipation Hepatology, Area 4C and constipation-predominant IBS. Dartmouth-Hitchcock Medical Center 1 Medical Center Drive Lebanon, NH 03756; Tel: 603-650-5215; Fax: 603-650-5225; onstipation is -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -
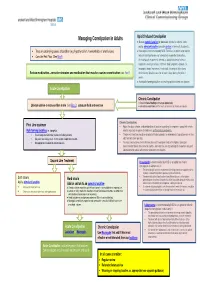
Managing Constipation in Adults
Managing Constipation in Adults Opio id Induced Constipation ● Use an osmotic laxative (or docusate which also softens stools and a stimulant laxative (consider dantron in terminally ill patients). • Treat any underlying causes of condition (e.g. hypothyroidism, haemorrhoids or anal fissures) ● Naloxegol is recommended by NICE TA345 as an option when opioid induced constipation has not adequately responded to laxatives. • Consider Red Flags (See Box A ) An inadequate response is defined as opioid-induced of at least moderate severity in at least 1of the 4 stool symptoms domain (i.e. incomplete bowel movement, hard stool, straining or false alarm) Review medication –consider stopping any medication that may be causing constipation (see Box B ) whilst taking1 laxative class for at least 4 days during the prior 2 weeks. ● Avoid bulk -forming l axatives for treating opioid induced constipation Acute Constipation Chronic Constipation ● Check for faecal loading and manage appropriately Lifestyle advise -to increase fibre in diet (see Box C ), adequate fluid and exercise ● Set realistic expectations for the results of treatment of chronic constipation. Chronic Constipation: First Line treatment • Adjust the dose, choice, and combination of laxative according to symptoms, speed with which Bulk forming laxatives i.e. ispaghula. relief is required, response to treatment, and individual preference. • Ensure adequate fluid intake (caution- frail elderly patients) • The dose of laxative should be gradually titrated upwards (or downwards) to produce one or two • May take several days to act- so not suitable if rapid relief required. soft, formed stools per day. • Not appropriate for opiod induced constipation. • If at least two laxatives (from different classes) have been tried at the highest tolerated recommended doses for at least 6 months, consider the use of prucalopride in women only and lubiprostone for adults with chronic idiopathic constipation. -

COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care
COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care In this issue: Glossary Page Chronic Constipation lasting longer than 3 months Introduction and background 1 constipation Drugs used in the management of Functional Chronic constipation without a known cause. Also known as primary 3 chronic constipation constipation constipation or idiopathic constipation Bulk-forming laxatives 4 Gastrocolic The occurrence of peristalsis following the entrance of food into the Osmotic laxatives 5 response empty stomach Stimulant laxatives 6 IBS Irritable Bowel Syndrome IBS-C Irritable Bowel Syndrome with Constipation Faecal softeners 7 Melanosis Dark brownish black pigmentation of the mucous membrane of the colon Peripheral opioid-receptor 7 coli due to the deposition of pigment in macrophages antagonists Myenteric Part of the enteric nervous system with an important role in regulating 5HT4 – receptor agonists 7 plexus gut motility Managing constipation in 8 NNT Number Needed to Treat pregnancy and breastfeeding OTC Over The Counter RCT Randomised Controlled Trial Secondary Constipation caused by a drug or medical condition. Secondary constipation constipation is also known as organic constipation SmPC Summary of Product Characteristics A twisting or looping of the bowel resulting in obstruction; can be life- Volvulus threatening Successful completion of the assessment questions at the end of this issue will provide you with 2 hours towards your CPD/CME requirements. Further copies of this and any other edition in the -

Chronic Constipation: an Evidence-Based Review
J Am Board Fam Med: first published as 10.3122/jabfm.2011.04.100272 on 7 July 2011. Downloaded from CLINICAL REVIEW Chronic Constipation: An Evidence-Based Review Lawrence Leung, MBBChir, FRACGP, FRCGP, Taylor Riutta, MD, Jyoti Kotecha, MPA, MRSC, and Walter Rosser MD, MRCGP, FCFP Background: Chronic constipation is a common condition seen in family practice among the elderly and women. There is no consensus regarding its exact definition, and it may be interpreted differently by physicians and patients. Physicians prescribe various treatments, and patients often adopt different over-the-counter remedies. Chronic constipation is either caused by slow colonic transit or pelvic floor dysfunction, and treatment differs accordingly. Methods: To update our knowledge of chronic constipation and its etiology and best-evidence treat- ment, information was synthesized from articles published in PubMed, EMBASE, and Cochrane Database of Systematic Reviews. Levels of evidence and recommendations were made according to the Strength of Recommendation taxonomy. Results: The standard advice of increasing dietary fibers, fluids, and exercise for relieving chronic constipation will only benefit patients with true deficiency. Biofeedback works best for constipation caused by pelvic floor dysfunction. Pharmacological agents increase bulk or water content in the bowel lumen or aim to stimulate bowel movements. Novel classes of compounds have emerged for treating chronic constipation, with promising clinical trial data. Finally, the link between senna abuse and colon cancer remains unsupported. Conclusions: Chronic constipation should be managed according to its etiology and guided by the best evidence-based treatment.(J Am Board Fam Med 2011;24:436–451.) copyright. Keywords: Chronic Constipation, Clinical Review, Evidence-Based Medicine, Family Medicine, Gastrointestinal Problems, Systematic Review The word “constipation” has varied meanings for was established in 1991 by Drossman et al, primar- different individuals.