Selection of Most Relevant Centrality Measures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Approximating Network Centrality Measures Using Node Embedding and Machine Learning
Approximating Network Centrality Measures Using Node Embedding and Machine Learning Matheus R. F. Mendon¸ca,Andr´eM. S. Barreto, and Artur Ziviani ∗† Abstract Extracting information from real-world large networks is a key challenge nowadays. For instance, computing a node centrality may become unfeasible depending on the intended centrality due to its computational cost. One solution is to develop fast methods capable of approximating network centralities. Here, we propose an approach for efficiently approximating node centralities for large networks using Neural Networks and Graph Embedding techniques. Our proposed model, entitled Network Centrality Approximation using Graph Embedding (NCA-GE), uses the adjacency matrix of a graph and a set of features for each node (here, we use only the degree) as input and computes the approximate desired centrality rank for every node. NCA-GE has a time complexity of O(jEj), E being the set of edges of a graph, making it suitable for large networks. NCA-GE also trains pretty fast, requiring only a set of a thousand small synthetic scale-free graphs (ranging from 100 to 1000 nodes each), and it works well for different node centralities, network sizes, and topologies. Finally, we compare our approach to the state-of-the-art method that approximates centrality ranks using the degree and eigenvector centralities as input, where we show that the NCA-GE outperforms the former in a variety of scenarios. 1 Introduction Networks are present in several real-world applications spread among different disciplines, such as biology, mathematics, sociology, and computer science, just to name a few. Therefore, network analysis is a crucial tool for extracting relevant information. -

Network Topologies Topologies
Network Topologies Topologies Logical Topologies A logical topology describes how the hosts access the medium and communicate on the network. The two most common types of logical topologies are broadcast and token passing. In a broadcast topology, a host broadcasts a message to all hosts on the same network segment. There is no order that hosts must follow to transmit data. Messages are sent on a First In, First Out (FIFO) basis. Token passing controls network access by passing an electronic token sequentially to each host. If a host wants to transmit data, the host adds the data and a destination address to the token, which is a specially-formatted frame. The token then travels to another host with the destination address. The destination host takes the data out of the frame. If a host has no data to send, the token is passed to another host. Physical Topologies A physical topology defines the way in which computers, printers, and other devices are connected to a network. The figure provides six physical topologies. Bus In a bus topology, each computer connects to a common cable. The cable connects one computer to the next, like a bus line going through a city. The cable has a small cap installed at the end called a terminator. The terminator prevents signals from bouncing back and causing network errors. Ring In a ring topology, hosts are connected in a physical ring or circle. Because the ring topology has no beginning or end, the cable is not terminated. A token travels around the ring stopping at each host. -

Topological Optimisation of Artificial Neural Networks for Financial Asset
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by LSE Theses Online The London School of Economics and Political Science Topological Optimisation of Artificial Neural Networks for Financial Asset Forecasting Shiye (Shane) He A thesis submitted to the Department of Management of the London School of Economics for the degree of Doctor of Philosophy. April 2015, London 1 Declaration I certify that the thesis I have presented for examination for the MPhil/PhD degree of the London School of Economics and Political Science is solely my own work other than where I have clearly indicated that it is the work of others (in which case the extent of any work carried out jointly by me and any other person is clearly identified in it). The copyright of this thesis rests with the author. Quotation from it is permitted, provided that full acknowledgement is made. This thesis may not be reproduced without the prior written consent of the author. I warrant that this authorization does not, to the best of my belief, infringe the rights of any third party. 2 Abstract The classical Artificial Neural Network (ANN) has a complete feed-forward topology, which is useful in some contexts but is not suited to applications where both the inputs and targets have very low signal-to-noise ratios, e.g. financial forecasting problems. This is because this topology implies a very large number of parameters (i.e. the model contains too many degrees of freedom) that leads to over fitting of both signals and noise. -

Closeness Centrality Extended to Unconnected Graphs : the Harmonic Centrality Index
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Infoscience - École polytechnique fédérale de Lausanne ASNA 2009 Zürich Closeness Centrality Extended To Unconnected Graphs : The Harmonic Centrality Index Yannick Rochat1 Institute of Applied Mathematics University of Lausanne, Switzerland [email protected] Abstract Social network analysis is a rapid expanding interdisciplinary field, growing from work of sociologists, physicists, historians, mathematicians, political scientists, etc. Some methods have been commonly accepted in spite of defects, perhaps because of the rareness of synthetic work like (Freeman, 1978; Faust & Wasserman, 1992). In this article, we propose an alternative index of closeness centrality defined on undirected networks. We show that results from its computation on real cases are identical to those of the closeness centrality index, with same computational complex- ity and we give some interpretations. An important property is its use in the case of unconnected networks. 1 Introduction The study of centrality is one of the most popular subject in the analysis of social networks. Determining the role of an individual within a society, its influence or the flows of information on which he can intervene are examples of applications of centrality indices. They are defined at an actor-level and are expected to compare and better understand roles of each individual in the net- work. In addition, a graph-level index called centralization (i.e. how much the index value of the most central node is bigger than the others) is defined for each existing index . Each index provides a way to highlight properties of individuals, depen- dently on its definition. -

Networking Solutions for Connecting Bluetooth Low Energy Devices - a Comparison
292 3 MATEC Web of Conferences , 0200 (2019) https://doi.org/10.1051/matecconf/201929202003 CSCC 2019 Networking solutions for connecting bluetooth low energy devices - a comparison 1,* 1 1 2 Mostafa Labib , Atef Ghalwash , Sarah Abdulkader , and Mohamed Elgazzar 1Faculty of Computers and Information, Helwan University, Egypt 2Vodafone Company, Egypt Abstract. The Bluetooth Low Energy (BLE) is an attractive solution for implementing low-cost, low power consumption, short-range wireless transmission technology and high flexibility wireless products,which working on standard coin-cell batteries for years. The original design of BLE is restricted to star topology networking, which limits network coverage and scalability. In contrast, other competing technologies like Wi-Fi and ZigBee overcome those constraints by supporting different topologies such as the tree and mesh network topologies. This paper presents a part of the researchers' efforts in designing solutions to enable BLE mesh networks and implements a tree network topology which is not supported in the standard BLE specifications. In addition, it discusses the advantages and drawbacks of the existing BLE network solutions. During analyzing the existing solutions, we highlight currently open issues such as flooding-based and routing-based solutions to allow end-to-end data transmission in a BLE mesh network and connecting BLE devices to the internet to support the Internet of Things (IoT). The approach proposed in this paper combines the default BLE star topology with the flooding based mesh topology to create a new hybrid network topology. The proposed approach can extend the network coverage without using any routing protocol. Keywords: Bluetooth Low Energy, Wireless Sensor Network, Industrial Wireless Mesh Network, BLE Mesh Network, Direct Acyclic Graph, Time Division Duplex, Time Division Multiple Access, Internet of Things. -
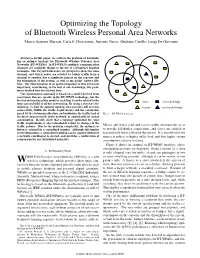
Optimizing the Topology of Bluetooth Wireless Personal Area Networks Marco Ajmone Marsan, Carla F
Optimizing the Topology of Bluetooth Wireless Personal Area Networks Marco Ajmone Marsan, Carla F. Chiasserini, Antonio Nucci, Giuliana Carello, Luigi De Giovanni Abstract— In this paper, we address the problem of determin- ing an optimal topology for Bluetooth Wireless Personal Area Networks (BT-WPANs). In BT-WPANs, multiple communication channels are available, thanks to the use of a frequency hopping technique. The way network nodes are grouped to share the same channel, and which nodes are selected to bridge traffic from a channel to another, has a significant impact on the capacity and the throughput of the system, as well as the nodes’ battery life- time. The determination of an optimal topology is thus extremely important; nevertheless, to the best of our knowledge, this prob- lem is tackled here for the first time. Our optimization approach is based on a model derived from constraints that are specific to the BT-WPAN technology, but the level of abstraction of the model is such that it can be related to the slave slave & bridge more general field of ad hoc networking. By using a min-max for- mulation, we find the optimal topology that provides full network master master & bridge connectivity, fulfills the traffic requirements and the constraints posed by the system specification, and minimizes the traffic load of Fig. 1. BT-WPAN topology. the most congested node in the network, or equivalently its energy consumption. Results show that a topology optimized for some traffic requirements is also remarkably robust to changes in the traffic pattern. Due to the problem complexity, the optimal so- Master and slaves send and receive traffic alternatively, so as lution is attained in a centralized manner. -

A Framework for Wireless Broadband Network for Connecting the Unconnected
A Framework for Wireless Broadband Network for Connecting the Unconnected Meghna Khaturia, Prasanna Chaporkar and Abhay Karandikar Department of Electrical Engineering, Indian Institute of Technology Bombay, Mumbai-400076 Email: fmeghnak,chaporkar,[email protected] Abstract—A significant barrier in providing affordable rural providing broadband in rural areas of India. Ashwini [6] broadband is to connect the rural and remote places to the optical and Aravind [7] are some other examples of rural network Point of Presence (PoP) over a distances of few kilometers. A testbeds deployed in India. Hop-Scotch is a long distance Wi- lot of work has been done in the area of long distance Wi- Fi networks. However, these networks require tall towers and Fi network based in UK [8]. LinkNet is a 52 node wireless high gain (directional) antennas. Also, they work in unlicensed network deployed in Zambia [9]. Also, there has been a band which has Effective Isotropically Radiated Power (EIRP) substantial research interest in designing the long distance Wi- limit (e.g. 1 W in India) which restricts the network design. In Fi based mesh networks for rural areas [10]–[12]. All these this work, we propose a Long Term Evolution-Advanced (LTE- efforts are based on IEEE 802.11 standard [13] in unlicensed A) network operating in TV UHF to connect the remote areas to the optical PoP. In India, around 100 MHz of TV UHF band i.e. 2:4 GHz or 5:8 GHz. When working with these band IV (470-585 MHz) is unused at any location and can frequency bands over a long distance point to point link, be put to an effective use in these areas [1]. -

Spectral Analysis of the Adjacency Matrix of Random Geometric Graphs
Spectral Analysis of the Adjacency Matrix of Random Geometric Graphs Mounia Hamidouche?, Laura Cottatellucciy, Konstantin Avrachenkov ? Departement of Communication Systems, EURECOM, Campus SophiaTech, 06410 Biot, France y Department of Electrical, Electronics, and Communication Engineering, FAU, 51098 Erlangen, Germany Inria, 2004 Route des Lucioles, 06902 Valbonne, France [email protected], [email protected], [email protected]. Abstract—In this article, we analyze the limiting eigen- multivariate statistics of high-dimensional data. In this case, value distribution (LED) of random geometric graphs the coordinates of the nodes can represent the attributes of (RGGs). The RGG is constructed by uniformly distribut- the data. Then, the metric imposed by the RGG depicts the ing n nodes on the d-dimensional torus Td ≡ [0; 1]d and similarity between the data. connecting two nodes if their `p-distance, p 2 [1; 1] is at In this work, the RGG is constructed by considering a most rn. In particular, we study the LED of the adjacency finite set Xn of n nodes, x1; :::; xn; distributed uniformly and matrix of RGGs in the connectivity regime, in which independently on the d-dimensional torus Td ≡ [0; 1]d. We the average vertex degree scales as log (n) or faster, i.e., choose a torus instead of a cube in order to avoid boundary Ω (log(n)). In the connectivity regime and under some effects. Given a geographical distance, rn > 0, we form conditions on the radius rn, we show that the LED of a graph by connecting two nodes xi; xj 2 Xn if their `p- the adjacency matrix of RGGs converges to the LED of distance, p 2 [1; 1] is at most rn, i.e., kxi − xjkp ≤ rn, the adjacency matrix of a deterministic geometric graph where k:kp is the `p-metric defined as (DGG) with nodes in a grid as n goes to infinity. -

Assortativity of Links in Directed Networks
Assortativity of links in directed networks Mahendra Piraveenan1, Kon Shing Kenneth Chung1, and Shahadat Uddin1 1Centre for Complex Systems Research, Faculty of Engineering and IT, The University of Sydney, NSW 2006, Australia Abstract— Assortativity is the tendency in networks where many technological and biological networks are slightly nodes connect with other nodes similar to themselves. De- disassortative, while most social networks, predictably, tend gree assortativity can be quantified as a Pearson correlation. to be assortative in terms of degrees [3], [5]. Recent work However, it is insufficient to explain assortative or disassor- defined degree assortativity for directed networks in terms tative tendencies of individual links, which may be contrary of in-degrees and out-degrees, and showed that an ensemble to the overall tendency in the network. In this paper we of definitions are possible in this case [6], [7]. define and analyse link assortativity in the context of directed It could be argued, however, that the overall assortativity networks. Using synthesised and real world networks, we tendency of a network may not be reflected by individual show that the overall assortativity of a network may be due links of the network. For example, it is possible that in a to the number of assortative or disassortative links it has, social network, which is overall assortative, some people the strength of such links, or a combination of both factors, may maintain their ‘fan-clubs’. In this situation, people who which may be reinforcing or opposing each other. We also have a great number of friends may be connected to people show that in some directed networks, link assortativity can who are less famous, or even loners. -

Wi-Fi® Mesh Networks: Discover New Wireless Paths
Wi-Fi® mesh networks: Discover new wireless paths Victor Asovsky WiLink™ 8 System Engineer Yaniv Machani WiLink 8 Software Team Leader Texas Instruments Table of Contents Abstract ...................................................................................................1 Introduction .............................................................................................1 Mesh network use case .........................................................................2 General capabilities ...............................................................................2 Range extension use case .....................................................................3 AP offloading use case ..........................................................................3 Wi-Fi mesh key features ........................................................................4 Homogenous ........................................................................................4 Self-forming ...........................................................................................4 Dynamic path selection .........................................................................4 Self-healing ...........................................................................................5 Possible issues in mesh networking ......................................................6 WLAN mesh deployment considerations .............................................7 Number of hops ....................................................................................7 -

Spatial Analysis and Modeling of Urban Transportation Networks
Spatial analysis and modeling of urban transportation networks Jingyi Lin Doctoral Thesis in Geodesy and Geoinformatics with Specialization in Geoinformatics Royal Institute of Technology Stockholm, Sweden, 2017 TRITA SoM 2017-15 ISRN KTH/SoM/2017-15/SE ISBN 978-91-7729-613-3 Doctoral Thesis Royal Institute of Technology (KTH) Department of Urban Planning and Environment Division of Geodesy and Geoinformatics SE-100 44 Stockholm Sweden © Jingyi Lin, 2017 Printed by US AB, Sweden 2017 Abstract Transport systems in general, and urban transportation systems in particular, are the backbone of a country or a city, therefore play an intrinsic role in the socio- economic development. There have been numerous studies on real transportation systems from multiple fileds, including geography, urban planning, and engineering. Geographers have extensively investigated transportation systems, and transport geography has developed as an important branch of geography with various studies on system structure, efficiency optimization, and flow distribution. However, the emergence of complex network theory provided a brand-new perspective for geographers and other researchers; therefore, it invoked more widespread interest in exploring transportation systems that present a typical node-link network structure. This trend inspires the author and, to a large extent, constitutes the motivation of this thesis. The overall objective of this thesis is to study and simulate the structure and dynamics of urban transportation systems, including aviation systems and ground transportation systems. More specificically, topological features, geometric properties, and dynamic evolution processes are explored and discussed in this thesis. To illustrate different construction mechanisms, as well as distinct evolving backgrounds of aviation systems and ground systems, China’s aviation network, U.S. -

A Centrality Measure for Urban Networks Based on the Eigenvector Centrality Concept
This is a repository copy of A Centrality Measure for Urban Networks Based on the Eigenvector Centrality Concept.. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/120372/ Version: Accepted Version Article: Agryzkov, Taras, Tortosa, Leandro, Vicent, Jose et al. (1 more author) (2017) A Centrality Measure for Urban Networks Based on the Eigenvector Centrality Concept. Environment and Planning B: Urban Analytics and City Science. ISSN 2399-8083 https://doi.org/10.1177/2399808317724444 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Journal Title A Centrality Measure for Urban XX(X):1–15 c The Author(s) 2015 Reprints and permission: Networks Based on the Eigenvector sagepub.co.uk/journalsPermissions.nav DOI: 10.1177/ToBeAssigned Centrality Concept www.sagepub.com/ Taras Agryzkov1 Leandro Tortosa1 Jose´ F. Vicent1 and Richard Wilson2 Abstract A massive amount of information as geo-referenced data is now emerging from the digitization of contemporary cities.