Lamont Et Al., 143 1991; Enright Et Al., 1998A,B)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PRINT \P Para "[ /Subtype /Document /Stpne Pdfmark "
__________________________________________________________________________________________ FLORA AND VEGETATION OF AVIVA LEASE AREA Prepared for: URS Australia Pty Ltd on behalf of Aviva Corporation Ltd Prepared by: Mattiske Consulting Pty Ltd February 2009 MATTISKE CONSULTING PTY LTD URS0808/195/08 MATTISKE CONSULTING PTY LTD __________________________________________________________________________________________ TABLE OF CONTENTS Page 1. SUMMARY ................................................................................................................................................ 1 2. INTRODUCTION ...................................................................................................................................... 3 2.1 Location .............................................................................................................................................. 3 2.2 Climate ................................................................................................................................................ 3 2.3 Landforms and Soils ........................................................................................................................... 4 2.4 Vegetation ........................................................................................................................................... 4 2.5 Declared Rare, Priority and Threatened Species ................................................................................. 4 2.6 Threatened Ecological Communities (TEC’s) ................................................................................... -

Ngaanyatjarra Central Ranges Indigenous Protected Area
PLAN OF MANAGEMENT for the NGAANYATJARRA LANDS INDIGENOUS PROTECTED AREA Ngaanyatjarra Council Land Management Unit August 2002 PLAN OF MANAGEMENT for the Ngaanyatjarra Lands Indigenous Protected Area Prepared by: Keith Noble People & Ecology on behalf of the: Ngaanyatjarra Land Management Unit August 2002 i Table of Contents Notes on Yarnangu Orthography .................................................................................................................................. iv Acknowledgements........................................................................................................................................................ v Cover photos .................................................................................................................................................................. v Abbreviations ................................................................................................................................................................. v Summary.................................................................................................................................................................................... 1 1 Introduction ....................................................................................................................................................................... 2 1.1 Background ............................................................................................................................................................... -

Their Botany, Essential Oils and Uses 6.86 MB
MELALEUCAS THEIR BOTANY, ESSENTIAL OILS AND USES Joseph J. Brophy, Lyndley A. Craven and John C. Doran MELALEUCAS THEIR BOTANY, ESSENTIAL OILS AND USES Joseph J. Brophy School of Chemistry, University of New South Wales Lyndley A. Craven Australian National Herbarium, CSIRO Plant Industry John C. Doran Australian Tree Seed Centre, CSIRO Plant Industry 2013 The Australian Centre for International Agricultural Research (ACIAR) was established in June 1982 by an Act of the Australian Parliament. ACIAR operates as part of Australia's international development cooperation program, with a mission to achieve more productive and sustainable agricultural systems, for the benefit of developing countries and Australia. It commissions collaborative research between Australian and developing-country researchers in areas where Australia has special research competence. It also administers Australia's contribution to the International Agricultural Research Centres. Where trade names are used this constitutes neither endorsement of nor discrimination against any product by ACIAR. ACIAR MONOGRAPH SERIES This series contains the results of original research supported by ACIAR, or material deemed relevant to ACIAR’s research and development objectives. The series is distributed internationally, with an emphasis on developing countries. © Australian Centre for International Agricultural Research (ACIAR) 2013 This work is copyright. Apart from any use as permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from ACIAR, GPO Box 1571, Canberra ACT 2601, Australia, [email protected] Brophy J.J., Craven L.A. and Doran J.C. 2013. Melaleucas: their botany, essential oils and uses. ACIAR Monograph No. 156. Australian Centre for International Agricultural Research: Canberra. -

Muelleria Layout Vol16 2002 13/12/02 12:28 PM Page 39
Muelleria layout Vol16 2002 13/12/02 12:28 PM Page 39 Muelleria 16: 39–42 (2002) Notes on Conothamnus Lindl. with the description of a new section, sect. Gongylocephalus Craven (Myrtaceae) L.A. Craven Australian National Herbarium, Centre for Plant Biodiversity Research, CSIRO Plant Industry, GPO Box 1600, Canberra, ACT 2601. [email protected] Abstract New morphological observations of Conothamnus Lindl. are reported and the new section, Gongylocephalus Craven, is described to accommodate Trichobasis Turcz. nom. illeg. which has been included in Conothamnus without taxonomic recognition until now. Introduction Conothamnus Lindl. was established by Lindley in 1839, based upon the single species C. trinervis Lindl. In 1852 Turczaninow described the genus Trichobasis Turcz. with T. aurea Turcz. its sole species. The name T. aurea is typified by the KW set of the collec- tion Drummond 5th coll. 147. Turczaninow’s generic name is illegitimate, being a later homonym of Trichobasis Léveillé, published in 1849. Bentham (1867) in effect trans- ferred Trichobasis aurea to the previously monotypic Conothamnus when he described C. divaricatus Benth. the name of which is typified by the K set of the type collection of T. aurea. In 1904 Diels added a third species to Conothamnus, C. neglectus Diels. All three species are restricted to the southwest of Western Australia. Conothamnus, including Trichobasis, has been treated as one of the several genera related to Melaleuca L., from which it has been distinguished by previous authors (e.g. Bentham 1867; Johnson & Briggs 1983; Rye 1987) by possession of a single ovule in each locule compared to several ovules per locule in Melaleuca. -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -

Inventory of Taxa for the Fitzgerald River National Park
Flora Survey of the Coastal Catchments and Ranges of the Fitzgerald River National Park 2013 Damien Rathbone Department of Environment and Conservation, South Coast Region, 120 Albany Hwy, Albany, 6330. USE OF THIS REPORT Information used in this report may be copied or reproduced for study, research or educational purposed, subject to inclusion of acknowledgement of the source. DISCLAIMER The author has made every effort to ensure the accuracy of the information used. However, the author and participating bodies take no responsibiliy for how this informrion is used subsequently by other and accepts no liability for a third parties use or reliance upon this report. CITATION Rathbone, DA. (2013) Flora Survey of the Coastal Catchments and Ranges of the Fitzgerald River National Park. Unpublished report. Department of Environment and Conservation, Western Australia. ACKNOWLEDGEMENTS The author would like to thank many people that provided valable assistance and input into the project. Sarah Barrett, Anita Barnett, Karen Rusten, Deon Utber, Sarah Comer, Charlotte Mueller, Jason Peters, Roger Cunningham, Chris Rathbone, Carol Ebbett and Janet Newell provided assisstance with fieldwork. Carol Wilkins, Rachel Meissner, Juliet Wege, Barbara Rye, Mike Hislop, Cate Tauss, Rob Davis, Greg Keighery, Nathan McQuoid and Marco Rossetto assissted with plant identification. Coralie Hortin, Karin Baker and many other members of the Albany Wildflower society helped with vouchering of plant specimens. 2 Contents Abstract .............................................................................................................................. -

Vegetation Monitoring – Swan Coastal Plain
2007 Vegetation Monitoring – Swan Coastal Plain 2007 Vegetation Monitoring - Swan Coastal Plain (Bunbury, Busselton-Capel Groundwater Areas) A Report to the DoW R. Loomes, J. Wilson & R. Froend Centre for Ecosystem Management ECU Joondalup CEM report no. 2007- 15 February 2008 Centre for Ecosystem Management 1 2007 Vegetation Monitoring – Swan Coastal Plain Table of Contents SUMMARY .................................................................................................................................................. 3 PROJECT CONTEXT ................................................................................................................................4 PROPOSED MONITORING PROGRAM................................................................................................ 5 BACKGROUND............................................................................................................................................ 5 MONITORING OBJECTIVES AND HYPOTHESES ............................................................................................ 6 PARAMETERS ........................................................................................................................................... 10 MONITORING FREQUENCY AND APPROACH ............................................................................................. 15 Transect establishment ....................................................................................................................... 15 Baseline Monitoring .......................................................................................................................... -

7008 Australian Native Plants Society Australia Hakea
FEBRUARY 20 10 ISSN0727 - 7008 AUSTRALIAN NATIVE PLANTS SOCIETY AUSTRALIA HAKEA STUDY GROUP NEWSLETTER NUMBER 42 Leader: Paul Kennedy PO Box 220 Strathmerton,Vic. 3 64 1 e mail: hakeaholic@,mpt.net.au Dear members. The last week of February is drawing to a close here at Strathrnerton and for once the summer season has been wetter and not so hot. We have had one very hot spell where the temperature reached the low forties in January but otherwise the maximum daily temperature has been around 35 degrees C. The good news is that we had 25mm of rain on new years day and a further 60mm early in February which has transformed the dry native grasses into a sea of green. The native plants have responded to the moisture by shedding that appearance of drooping lack lustre leaves to one of bright shiny leaves and even new growth in some cases. Many inland parts of Queensland and NSW have received flooding rains and hopefully this is the signal that the long drought is finally coming to an end. To see the Darling River in flood and the billabongs full of water will enable regeneration of plants, and enable birds and fish to multiply. Unfortunately the upper reaches of the Murray and Murrurnbidgee river systems have missed out on these flooding rains. Cliff Wallis from Merimbula has sent me an updated report on the progress of his Hakea collection and was complaining about the dry conditions. Recently they had about 250mm over a couple of days, so I hope the species from dryer areas are not sitting in waterlogged soil. -

Detailed Flora and Vegetation Assessment
Appendix C: Detailed Flora and Vegetation Assessment. METRONET Morley-Ellenbrook line (RPS 2019) MEL-MNO-RPS-EN-REF-0018_B DETAILED FLORA AND VEGETATION ASSESSMENT METRONET Morley-Ellenbrook line EEL17158.005 Detailed flora and vegetation assessment Rev 0 14 June 2019 rpsgroup.com REPORT Document status Version Purpose of document Authored by Reviewed by Approved by Review date Draft A Draft for client review CarGil GilGla NA 30/01/2019 Rev 0 Final for issue CarGil/JulHan GilGla SteRol 14/06/2019 Approval for issue SteRol 14 June 2019 This report was prepared by RPS within the terms of RPS’ engagement with its client and in direct response to a scope of services. This report is supplied for the sole and specific purpose for use by RPS’ client. The report does not account for any changes relating the subject matter of the report, or any legislative or regulatory changes that have occurred since the report was produced and that may affect the report. RPS does not accept any responsibility or liability for loss whatsoever to any third party caused by, related to or arising out of any use or reliance on the report. Prepared by: Prepared for: RPS METRONET Caroline Gill Managing Scientist Level 2, 27-31 Troode Street 140 William Street West Perth WA 6005 PERTH WA 6000 T +61 8 9211 1111 E [email protected] EEL17158.005 | Detailed flora and vegetation assessment | Rev 0 | 14 June 2019 rpsgroup.com Page ii REPORT Contents Summary ........................................................................................................................................................... 1 Survey objectives and scope of works ..................................................................................................... 1 Detailed flora and vegetation survey findings ......................................................................................... -
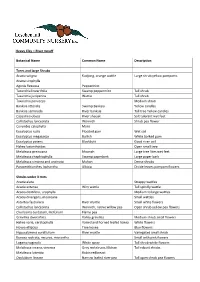
Heavy Clay – River Runoff Botanical Name Common Name Description Trees and Large Shrubs Acacia Saligna Kudjong, Orange Wattle
Heavy Clay – River runoff Botanical Name Common Name Description Trees and large Shrubs Acacia saligna Kudjong, orange wattle Large shrub yellow pompoms Acacia urophylla Agonis flexuosa Peppermint Taxandria linearifolia Swamp peppermint Tall shrub Taxandria juniperina Wattie Tall shrub Taxandria parviceps Medium shrub Banksia littoralis Swamp Banksia Yellow candles Banksia seminuda River banksia Tall tree Yellow candles Casuarina obesa River sheoak Salt tolerant wet feet Callistachys lanceolata Wonnich Shrub pea flower Corymbia calophylla Marri Eucalyptus rudis Flooded gum Wet soil Eucalyptus megacarpa Bullich White barked gum Eucalyptus patens Blackbutt Good river soil Hakea lasianthoides Open small tree Melaleuca preissiana Moonah Large tree likes wet feet Melaleuca rhaphiophylla Swamp paperbark Large paper bark Melaleuca viminea and uncinata Mohan Dense shrubs Paraserithianthes lophantha Albizia Divide leaves pompom flowers Shrubs under 3 mtrs Acacia alata Strappy wattles Acacia extensa Wiry wattle Tall spindly wattle Acacia dentifera, urophylla Medium to large wattles Acacia divergens, mooreana Small wattles Astartea fasciularis River myrtle Small white flowers Callistachys lanceolata Wonnich, native willow pea Open shrub yellow pea flowers Chorizema cordatum, ilicifolium Flame pea Grevillea diversifolia Valley grevillea Medium shrub small flowers Hakea varia, ceratophylla Varied and horned leafed hakea White flowers Hovea elliptica Tree hovea Blue flowers Hypocalymma cordifolium River myrtle Variegated small shrub Kunzea rostrata, -

Petrophile Shirleyae (Proteaceae): Peter M
A U S T R AL I A N N A T I V E P L A N T S S O C I E T Y ( A U S T R A L I A ) Isopogon & Petrophile Study Group Newsletter No. 25 November 2019 ISSN 1445-9493 Website http://anpsa.org.au/iso-petSG/ STUDY GROUP LEADERS/NEWSLETTER EDITORS Catriona Bate & Phil Trickett Email: [email protected] Ph: 0409 789 567 Isopogon ‘Coaldale Cracker’ in cultivation, Little Forest NSW. See our articles in this issue. Back issues of the Isopogon & Petrophile Study Group Newsletter are available at http://anpsa.org.au/iso-petSG/IPSG-news.html Isopogon & Petrophile Study Group Newsletter 25, November 2019 1 In this issue Editorial From our members Exchanging cuttings and seed Isopogon uncinatus makes Top 100 Update on current threats to isopogons and petrophiles Petrophile latericola stamp sows the seeds of conservation Introducing a new hybrid Finding ‘Coaldale Cracker’ Isopogons through insect eyes Northern exposure Isopogon profile – Isopogon linearis Petrophile profile – Petrophile axillaris Species that mound – Dryandras, Petrophile filifolia and Isopogon villosus: Margaret Pieroni Species named for people On the Etymology of Petrophile shirleyae (Proteaceae): Peter M. Olde Petrophile shirleyae (conesticks): Allan Carr Interstock grafting WA spring report 2019 Turning heads in the bush In the press Financial report Hi fellow Isophiles, Firstly, we wish to thank everyone for their generous feedback on THE PETROPHILE ISSUE, focusing on the poor relation of I & Ps, the petrophiles. We feel they are very under-appreciated and that once we learn to propagate and successfully grow some of the many spectacular species, that will all change. -

Flora and Vegetation Of
__________________________________________________________________________________________ FLORA AND VEGETATION OF AVIVA LEASE AREA Prepared for: URS Australia Pty Ltd on behalf of Aviva Corporation Ltd Prepared by: Mattiske Consulting Pty Ltd February 2009 MATTISKE CONSULTING PTY LTD URS0808/195/08 MATTISKE CONSULTING PTY LTD __________________________________________________________________________________________ TABLE OF CONTENTS Page 1. SUMMARY ................................................................................................................................................ 1 2. INTRODUCTION ...................................................................................................................................... 3 2.1 Location .............................................................................................................................................. 3 2.2 Climate ................................................................................................................................................ 3 2.3 Landforms and Soils ........................................................................................................................... 4 2.4 Vegetation ........................................................................................................................................... 4 2.5 Declared Rare, Priority and Threatened Species ................................................................................. 4 2.6 Threatened Ecological Communities (TEC’s) ...................................................................................