Solute Sources and Geochemical Processes in Subglacial Lake Whillans, West Antarctica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geochemistry of Subglacial Lake Whillans, West Antarctica: Implications for Microbial Activity
5th International Conference on Polar & Alpine Microbiology, Big Sky, MT, USA, 2013 Geochemistry of subglacial Lake Whillans, West Antarctica: Implications for microbial activity. Mark Skidmore1, Andrew Mitchell2, Carlo Barbante3, Alex Michaud4, Trista Vick-Majors4, John Priscu4 1Earth Sciences, Montana State University, Bozeman, MT, USA, 2Geography and Earth Sciences, Aberystwyth University, Aberystwyth, UK, 3Institute for the Dynamics of Environmental Processes-CNR, University of Venice, Venice, Italy. 4Land Resources and Environmental Sciences, Montana State University, Bozeman, MT, USA. Subglacial Lake Whillans is located beneath the Whillans Ice Stream in West Antarctica. The lake is situated beneath 800 m of ice and ~ 70 km upstream of the grounding line where Whillans Ice Stream terminates into the Ross Sea. Water and sediment samples were recovered from the lake, using clean access drilling technologies, in January, 2013. Isotopic analysis of the lake waters indicates basal meltwater from the ice sheet as the dominant water source. Geochemical analysis of the lake waters reveal it is freshwater with total dissolved solids concentrations about 1/70th that of sea water. However, mineral weathering is a significant source of solute to the lake water with a contribution also from sea water. Nutrients N and P are present at micromolar concentrations. The sediment porewaters from shallow cores (~ 40 cm depth) of the subglacial lake sediments indicate increasing solute concentration with depth, with up to ~ five times greater solute concentrations than in the lake waters. Collectively the aqueous geochemistry indicates an environment favorable for microbial activity. Thus, microbially-driven mineral weathering appears likely beneath the Whillans Ice Stream, as has been demonstrated in other subglacial systems, including in subglacial sediments of the neighboring Kamb Ice Stream. -

7Th Grade February Break Packet.Pdf
__________________ ___________________ _________________ Read this story. Then answer questions 15 through 21. e narrator, Holling Hoodhood, has a crush on Meryl Lee Kowalski. Holling’s father has been honored earlier in the story by a local business group as the best businessman of 1967. Excerpt from e Wednesday Wars by Gary D. Schmidt 1 e following week the school board met to decide on the model for the new junior high school—which was probably why Mr. Kowalski had been spending all his time muttering “classical, classical, classical.” e meeting was to be at four o’clock in the high school administration building. Mr. Kowalski would present his plan and model, and then my father would present his plan and model, and then the school board would meet in private session to decide whether Kowalski and Associates or Hoodhood and Associates would be the architect for the new junior high school. 2 I know all of this because my father was making me come. It was time I started to learn the business, he said. I needed to see firsthand how competitive bidding worked. I needed to experience architectural presentations. I needed to see architecture as the blood sport that it truly was. 3 e meeting was in the public conference room, and when I got there aer school, the school board members were all sitting at the head table, studying the folders with architectural bids. Mr. Kowalski and my father were sitting at two of the high school desks—which made the whole thing seem a little weirder than it needed to be. -

Antarctic Primer
Antarctic Primer By Nigel Sitwell, Tom Ritchie & Gary Miller By Nigel Sitwell, Tom Ritchie & Gary Miller Designed by: Olivia Young, Aurora Expeditions October 2018 Cover image © I.Tortosa Morgan Suite 12, Level 2 35 Buckingham Street Surry Hills, Sydney NSW 2010, Australia To anyone who goes to the Antarctic, there is a tremendous appeal, an unparalleled combination of grandeur, beauty, vastness, loneliness, and malevolence —all of which sound terribly melodramatic — but which truly convey the actual feeling of Antarctica. Where else in the world are all of these descriptions really true? —Captain T.L.M. Sunter, ‘The Antarctic Century Newsletter ANTARCTIC PRIMER 2018 | 3 CONTENTS I. CONSERVING ANTARCTICA Guidance for Visitors to the Antarctic Antarctica’s Historic Heritage South Georgia Biosecurity II. THE PHYSICAL ENVIRONMENT Antarctica The Southern Ocean The Continent Climate Atmospheric Phenomena The Ozone Hole Climate Change Sea Ice The Antarctic Ice Cap Icebergs A Short Glossary of Ice Terms III. THE BIOLOGICAL ENVIRONMENT Life in Antarctica Adapting to the Cold The Kingdom of Krill IV. THE WILDLIFE Antarctic Squids Antarctic Fishes Antarctic Birds Antarctic Seals Antarctic Whales 4 AURORA EXPEDITIONS | Pioneering expedition travel to the heart of nature. CONTENTS V. EXPLORERS AND SCIENTISTS The Exploration of Antarctica The Antarctic Treaty VI. PLACES YOU MAY VISIT South Shetland Islands Antarctic Peninsula Weddell Sea South Orkney Islands South Georgia The Falkland Islands South Sandwich Islands The Historic Ross Sea Sector Commonwealth Bay VII. FURTHER READING VIII. WILDLIFE CHECKLISTS ANTARCTIC PRIMER 2018 | 5 Adélie penguins in the Antarctic Peninsula I. CONSERVING ANTARCTICA Antarctica is the largest wilderness area on earth, a place that must be preserved in its present, virtually pristine state. -

Race Is on to Find Life Under Antarctic Ice Russia, U.S., and Britain Drill to Discover Microbes in Subglacial Lakes
Race Is On to Find Life Under Antarctic Ice Russia, U.S., and Britain drill to discover microbes in subglacial lakes. A person works at the drilling site atop Lake Ellsworth, Antarctica. Photograph courtesy Pete Bucktrout, British Antarctic Survey Marc Kaufman for National Geographic News Published December 18, 2012 A hundred years ago, two teams of explorers set out to be the first people ever to reach the South Pole. The race between Roald Amundsen of Norway and Robert Falcon Scott of Britain became the stuff of triumph, tragedy, and legend. (See rare pictures of Scott's expedition.) Today, another Antarctic drama is underway that has a similar daring and intensity—but very different stakes. Three unprecedented, major expeditions are underway to drill deep through the ice covering the continent and, researchers hope, penetrate three subglacial lakes not even known to exist until recently. The three players—Russia, Britain, and the United States—are all on the ice now and are in varying stages of their preparations. The first drilling was attempted last week by the British team at Lake Ellsworth, but mechanical problems soon cropped up in the unforgiving Antarctic cold, putting a temporary hold on their work. The key scientific goal of the missions: to discover and identify living organisms in Antarctica's dark, pristine, and hidden recesses. (See"Antarctica May Contain 'Oasis of Life.'") Scientists believe the lakes may well be home to the kind of "extreme" life that could eke out an existence on other planets or moons of our solar system, so finding them on Earth could help significantly in the search for life elsewhere. -

Environment and Processes of Subglacial Lake Whillans, West Antarctica
Environment and Processes of Subglacial Lake Whillans, West Antarctica Ross Powell1, Tim Hodson1, Jeremy Wei1, Slawek Tulaczyk2, Stefanie Brachfeld3, Isla Castañeda4, Rebecca Putkammer1, Reed Scherer1, and the WISSARD Science Team 1Department of Geology and Environmental Geosciences, Northern Illinois University 2Department of Earth and Planetary Sciences, University of California Santa Cruz 3Earth and Environmental Studies, Montclair State University 4Department of Geosciences, University of Massachusetts, Amherst Subglacial Lake Whillans (SLW) lies 800m below the low gradient, Whillans Ice Plain West Antarctica, upstream from where Whillans Ice Stream goes afloat into the Ross Ice Shelf. During 2013-14 season the WISSARD project made measurements in, and collected water and sediment samples from SLW. Sediment is typical subglacial till; a homogenized, structureless diamicton. Debris from local basal ice is likely not contributed to SLW by rainout because ice is theoretically below pressure melting. So lake floor diamicton likely was transported to SLW by deformation while the ice stream was grounded at the drill site both prior to lake formation and during lake “lowstands”. Satellite altimetry has shown SLW experiences short (~7 month) discharge events, lowering the ice surface and lake water level by between 1- 4m. Lake lowstands are separated by longer periods of gradual recharge, but over the period of many lowstands the ice stream is suspected to touch down and couple with the lake floor, potentially shearing new till into SLW. Subglacial hydrological diversions also may play a role in SLW history; if water is captured by another drainage basin, then the bed at SLW will also act as a till. The lack of sorted sediment (apart from a lamina of mud at the sediment-water interface) and erosional lags within sediment cores indicate water flow during discharge/recharge events has had a low current velocity with quiescent conditions in the lake. -
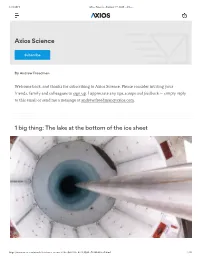
1 Big Thing: the Lake at the Bottom of the Ice Sheet
1/21/2019 Axios Science - January 17, 2019 - Axios Axios Science Subscribe By Andrew Freedman Welcome back, and thanks for subscribing to Axios Science. Please consider inviting your friends, family and colleagues to sign up. I appreciate any tips, scoops and feedback — simply reply to this email or send me a message at [email protected]. 1 big thing: The lake at the bottom of the ice sheet https://www.axios.com/newsletters/axios-science-616ee9dd-0f6e-411f-9994-e78368d62cd5.html 1/10 1/21/2019 Axios Science - January 17, 2019 - Axios The UV collar and borehole, showing the water of Subglacial Lake Mercer, from the SALSA Project. Photo: Billy Collins, SALSA Science Team A research team drilling thousands of feet under the Antarctic ice sheet has found new evidence of microbial life there — life forms that live in an environment devoid of sunlight and are not known to exist elsewhere. Why it matters: Subglacial Lake Mercer is only the second subglacial lake in Antarctica to be explored, in an area as vast as twice the continental United States. That means scientists have to draw a lot of conclusions from just two holes — but it's the only way to learn about what kind of life exists in the mysterious world of lakes and rivers deep beneath the ice. In addition, researchers think the water moving underneath the ice sheet could further destabilize portions of it, adding to the already quickening pace of sea level rise. Details: Researchers sampled Subglacial Lake Whillans in 2013, finding abundant microbial life. Now we have much more data collected from Subglacial Lake Mercer. -

Did Holocene Climate Changes Drive West Antarctic Grounding Line Retreat and Re-Advance?
https://doi.org/10.5194/tc-2020-308 Preprint. Discussion started: 19 November 2020 c Author(s) 2020. CC BY 4.0 License. Did Holocene climate changes drive West Antarctic grounding line retreat and re-advance? Sarah U. Neuhaus1, Slawek M. Tulaczyk1, Nathan D. Stansell2, Jason J. Coenen2, Reed P. Scherer2, Jill 5 A. Mikucki3, Ross D. Powell2 1Earth and Planetary Sciences, University oF CaliFornia Santa Cruz, Santa Cruz, CA, 95064, USA 2Department oF Geology and Environmental Geosciences, Northern Illinois University, DeKalb, IL, 60115, USA 3Department oF Microbiology, University oF Tennessee Knoxville, Knoxville, TN, 37996, USA 10 Correspondence to: Sarah U. Neuhaus ([email protected]) Abstract. Knowledge oF past ice sheet conFigurations is useFul For informing projections of Future ice sheet dynamics and For calibrating ice sheet models. The topology oF grounding line retreat in the Ross Sea Sector oF Antarctica has been much debated, but it has generally been assumed that the modern ice sheet is as small as it has been for more than 100,000 years 15 (Conway et al., 1999; Lee et al., 2017; Lowry et al., 2019; McKay et al., 2016; Scherer et al., 1998). Recent findings suggest that the West Antarctic Ice Sheet (WAIS) grounding line retreated beyond its current location earlier in the Holocene and subsequently re-advanced to reach its modern position (Bradley et al., 2015; Kingslake et al., 2018). Here, we further constrain the post-LGM grounding line retreat and re-advance in the Ross Sea Sector using a two-phase model of radiocarbon input and decay in subglacial sediments From six sub-ice sampling locations. -

WISSARD at Subglacial Lake Whillans, West Antarctica: Scientific Operations and Initial Observations
Annals of Glaciology 55(65) 2014 doi: 10.3189/2014AoG65A009 1 WISSARD at Subglacial Lake Whillans, West Antarctica: scientific operations and initial observations Slawek TULACZYK,1 Jill A. MIKUCKI,2 Matthew R. SIEGFRIED,3 John C. PRISCU,4 C. Grace BARCHECK,1 Lucas H. BEEM,1 Alberto BEHAR,5 Justin BURNETT,6 Brent C. CHRISTNER,7 Andrew T. FISHER,1 Helen A. FRICKER,3 Kenneth D. MANKOFF,1 Ross D. POWELL,8 Frank RACK,6 Daniel SAMPSON,1 Reed P. SCHERER,8 Susan Y. SCHWARTZ1 and THE WISSARD SCIENCE TEAM9 1Department of Earth and Planetary Sciences, University of California Santa Cruz, Santa Cruz, CA, USA E-mail: [email protected] 2Department of Microbiology, University of Tennessee, Knoxville, TN, USA 3Institute of Geophysics and Planetary Physics, Scripps Institution of Oceanography, University of California, San Diego, La Jolla, CA, USA 4Department of Land Resources and Environmental Science, Montana State University, Bozeman, MT, USA 5Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA, USA 6Antarctic Geological Drilling Science Management Office, University of Nebraska, Lincoln, NE, USA 7Department of Biological Sciences, Louisiana State University, Baton Rouge, LA, USA 8Geology and Environmental Geosciences, Northern Illinois University, DeKalb, IL, USA 9Full personnel list at http://www.wissard.org/about/wissard-personnel ABSTRACT. A clean hot-water drill was used to gain access to Subglacial Lake Whillans (SLW) in late January 2013 as part of the Whillans Ice Stream Subglacial Access Research Drilling (WISSARD) project. Over 3 days, we deployed an array of scientific tools through the SLW borehole: a downhole camera, a conductivity±temperature±depth (CTD) probe, a Niskin water sampler, an in situ filtration unit, three different sediment corers, a geothermal probe and a geophysical sensor string. -

Under the Ice: Studying One of the Last Unexplored Aquatic Environments on Earth
LIVE INTERACTIVE LEARNING @ YOUR DESKTOP Under The Ice: Studying one of the last unexplored aquatic environments on Earth Presented by: Dr. Slawek Tulaczyk and Dr. Brent Christner Thursday, May 27, 2010 Under the Ice: Studying one of the last unexplored aquatic environments on Earth Prof. Slawek Tulaczyk Department of Earth and Planetary Sciences University of California, Santa Cruz, CA, USA Old view (book titles): “The Crystal Desert” “Big Dead Place” Ice base seen from a subglacial water cavity below a mile of ice The largest wetland on Earth surrounding lakes and rivers (NSF images) Was Antarctica always covered by ice? Yes No When did Antarctica become covered by ice? (A) 30,000 years ago (B) 300,000 years ago (C) 3 mln years ago (D) 30 mln years ago (E) 300 mln years ago Let’s Pause for Questions Where does the water come from? Ice sheet melts at its base (Llubes et al., 2006) What are sources of heat beneath ice? (A) Sunlight (B) Radioactive heat (C) Geothermal heat (D) Frictional heating during motion Water is being squeezed towards the ocean but gets trapped in lakes The largest subglacial lake in Antarctica is Lake Vostok Some lakes are active Subglacial Lake Whillans has drained twice drainage drainage >100 active subglacial lakes mapped out in 2003-08 Ice velocity map from Dr. Ian Joughin, UW red ~1000 of m/yr, blue ~100 m/yr, yellowish-green ~10 m/yr mass gain mass loss snowfall iceberg calving surface melt basal melt Why are Antarctic subglacial lakes worth studying? (A) They may be microbial habitats (B) Subglacial water -

Microbial Community Structure of Subglacial Lake Whillans, West Antarctica
fmicb-07-01457 September 22, 2016 Time: 12:35 # 1 ORIGINAL RESEARCH published: 22 September 2016 doi: 10.3389/fmicb.2016.01457 Microbial Community Structure of Subglacial Lake Whillans, West Antarctica Amanda M. Achberger1, Brent C. Christner1,2,3*, Alexander B. Michaud4†, John C. Priscu4, Mark L. Skidmore5, Trista J. Vick-Majors4 and the WISSARD Science Team‡ 1 Department of Biological Sciences, Louisiana State University, Baton Rouge, LA, USA, 2 Department of Microbiology and Edited by: Cell Science, University of Florida, Gainesville, FL, USA, 3 Biodiversity Institute, University of Florida, Gainesville, FL, USA, Michael Rappe, 4 Department of Land Resources and Environmental Science, Montana State University, Bozeman, MT, USA, 5 Department University of Hawaii at Manoa, USA of Earth Sciences, Montana State University, Bozeman, MT, USA Reviewed by: Brandi K. Reese, Texas A&M University–Corpus Christi, Subglacial Lake Whillans (SLW) is located beneath ∼800 m of ice on the Whillans Ice USA Stream in West Antarctica and was sampled in January of 2013, providing the first Nathalie Pradel, Institute of Research opportunity to directly examine water and sediments from an Antarctic subglacial lake. for Development, France To minimize the introduction of surface contaminants to SLW during its exploration, an *Correspondence: access borehole was created using a microbiologically clean hot water drill designed to Brent C. Christner reduce the number and viability of microorganisms in the drilling water. Analysis of 16S xner@ufl.edu rRNA genes (rDNA) amplified from samples of the drilling and borehole water allowed †Present address: Alexander B. Michaud, an evaluation of the efficacy of this approach and enabled a confident assessment Center for Geomicrobiology, of the SLW ecosystem inhabitants. -

IUCN Global Ecosystem Typology 2.0 Descriptive Profiles for Biomes and Ecosystem Functional Groups
IUCN Global Ecosystem Typology 2.0 Descriptive profiles for biomes and ecosystem functional groups David A. Keith, Jose R. Ferrer-Paris, Emily Nicholson and Richard T. Kingsford (editors) INTERNATIONAL UNION FOR CONSERVATION OF NATURE About IUCN IUCN is a membership Union uniquely composed of both government and civil society organisations. It provides public, private and non-governmental organisations with the knowledge and tools that enable human progress, economic development and nature conservation to take place together. Created in 1948, IUCN is now the world’s largest and most diverse environmental network, harnessing the knowledge, resources and reach of more than 1,400 Member organisations and some 15,000 experts. It is a leading provider of conservation data, assessments and analysis. Its broad membership enables IUCN to fill the role of incubator and trusted repository of best practices, tools and international standards. IUCN provides a neutral space in which diverse stakeholders including governments, NGOs, scientists, businesses, local communities, indigenous peoples organisations and others can work together to forge and implement solutions to environmental challenges and achieve sustainable development. www.iucn.org https://twitter.com/IUCN/ About the Commission on Ecosystem Management (CEM) The Commission on Ecosystem Management (CEM) promotes ecosystem-based approaches for the management of landscapes and seascapes, provides guidance and support for ecosystem-based management and promotes resilient socio-ecological systems -

Subglacial Lake Whillans Microbial Biogeochemistry: a Synthesis Of
Subglacial Lake Whillans microbial biogeochemistry: a rsta.royalsocietypublishing.org synthesis of current knowledge J. A. Mikucki1,2,P.A.Lee3,D.Ghosh1,A.M.Purcell1, Discussion A. C. Mitchell4,K.D.Mankoff5,A.T.Fisher6, 6 7 7 7 Cite this article: Mikucki JA et al.2016 S. Tulaczyk ,S.Carter, M. R. Siegfried ,H.A.Fricker, Subglacial Lake Whillans microbial T. Hodson8, J. Coenen8,R.Powell8,R.Scherer8, biogeochemistry: a synthesis of current 9 10 10 knowledge. Phil.Trans.R.Soc.A374: 20140290. T. Vick-Majors ,A.A.Achberger ,B.C.Christner , http://dx.doi.org/10.1098/rsta.2014.0290 M. Tranter11 and the WISSARD Science Team Accepted: 2 October 2015 1Department of Microbiology, University of Tennessee, Knoxville, TN 37996, USA One contribution of 17 to a Theo Murphy 2Department of Biology, Middlebury College, Middlebury, meeting issue ‘Antarctic subglacial lake VT 05753, USA exploration: first results and future plans’. 3Hollings Marine Lab, College of Charleston, Charleston, SC 29412, USA Subject Areas: 4 biogeochemistry, glaciology Department of Geography and Earth Sciences, Aberystwyth University, Aberystwyth, UK Keywords: 5Department of Geosciences, The Pennsylvania State University, Antarctica, subglacial lakes, microbial University Park, PA 16802, USA diversity, geomicrobiology, Whillans Ice 6Earth and Planetary Sciences, University of California, Santa Cruz, Stream, WISSARD CA, USA 7Institute for Geophysics and Planetary Physics, Scripps Institution Author for correspondence: of Oceanography, University of California, San Diego, CA 92093,