Structure and Variability in Human Tongue Muscle Anatomy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mouth Esophagus Stomach Rectum and Anus Large Intestine Small
1 Liver The liver produces bile, which aids in digestion of fats through a dissolving process known as emulsification. In this process, bile secreted into the small intestine 4 combines with large drops of liquid fat to form Healthy tiny molecular-sized spheres. Within these spheres (micelles), pancreatic enzymes can break down fat (triglycerides) into free fatty acids. Pancreas Digestion The pancreas not only regulates blood glucose 2 levels through production of insulin, but it also manufactures enzymes necessary to break complex The digestive system consists of a long tube (alimen- 5 carbohydrates down into simple sugars (sucrases), tary canal) that varies in shape and purpose as it winds proteins into individual amino acids (proteases), and its way through the body from the mouth to the anus fats into free fatty acids (lipase). These enzymes are (see diagram). The size and shape of the digestive tract secreted into the small intestine. varies in each individual (e.g., age, size, gender, and disease state). The upper part of the GI tract includes the mouth, throat (pharynx), esophagus, and stomach. The lower Gallbladder part includes the small intestine, large intestine, The gallbladder stores bile produced in the liver appendix, and rectum. While not part of the alimentary 6 and releases it into the duodenum in varying canal, the liver, pancreas, and gallbladder are all organs concentrations. that are vital to healthy digestion. 3 Small Intestine Mouth Within the small intestine, millions of tiny finger-like When food enters the mouth, chewing breaks it 4 protrusions called villi, which are covered in hair-like down and mixes it with saliva, thus beginning the first 5 protrusions called microvilli, aid in absorption of of many steps in the digestive process. -

Tongue -Tie (Ankyloglossia) and Lip -Tie (Lip Adhesion)
Tongue -Tie (Ankyloglossia) and Lip -Tie (Lip Adhesion) What is Tongue-Tie? Most of us think of tongue -tie as a situation we find ourselves in when we are too excited to speak. Actually, tongue- tie is the non medical term for a relatively common physical condition that limits the use of the tongue, ankyloglossia. Lip -tie is a condition where the upper lip cannot be curled or moved normally. Before we are born, a strong cord of tissue that guides development of mouth structures is positioned in the center of the mouth. It is called a frenulum. As we develop, this frenulum recedes and thins. The lingual (tongue) or labial (lip) frenulum is visible and easily felt if you look in the mirror under your tongue and lip. In some children, the frenulum is especially tight or fails to recede and may cause tongue/lip mobility problems. The tongue and lip are a very complex group of muscles and are important for all oral function. For this reason having tongue tie can lead to nursing, eating, dental, or speech problems, which may be serious in some individuals. When Is Tongue and Lip- Tie a Problem That Needs Treatment? Infants A new baby with a too tight tongue and/or lip frenulum can have trouble sucking and may have poor weight gain. If they cannot make a good seal on the nipple, they may swallow air causing gas and stomach problems. Such feeding problems should be discussed with Dr. Sierra. Nursing mothers who experience significant pain while nursing or whose baby has trouble latching on should have their child evaluated for tongue and lip tie. -

Clinical Review Nursingingeneralpractice
The health benefits of nose breathing Item Type Article Authors Allen, Ruth Publisher Nursing in General Practice Journal Nursing in General Practice Download date 01/10/2021 07:15:20 Link to Item http://hdl.handle.net/10147/559021 Find this and similar works at - http://www.lenus.ie/hse clinical review nursingingeneralpractice The health benefits of nose breathing DR Alan RUth, BehaviouRal Medicine PRactitioneR “For breath is life, and if you breathe well you will live long on earth.” sanskrit Proverb For the most part people are unaware of their breathing and take it for granted that they do it correctly. t has been estimated that approximately one third of people ing. However, it has been estimated that up to 30-50% of modern don’t breathe well enough to sustain normal health. These adults breathe through the mouth, especially during the early people do not get enough oxygenation of their cells, tissues morning hours. and organs. In the book Behavioural and Psychological Ap- Mouth breathing is common in individuals whose nasal proaches to Breathing Disorders, Dr Chandra Patel describes passages are blocked or restricted. A deviated nasal septum Ithe problem with breathing as follows: or small nostril size can lead a person to breathe through their “We start life with a breath, and the process continues mouth instead of their nose. However, breathing through the automatically for the rest of our lives. Because breathing mouth most of the time was not nature’s intention. Many studies continues on its own, without our awareness, it does not have demonstrated that chronic mouth breathing can result in a necessarily mean that it is always functioning for optimum number of adverse health consequences (see Table 1). -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Chief Complaint: "Swelling of Tongue and Difficulty Breathing and Swallowing"
Chief Complaint: "swelling of tongue and difficulty breathing and swallowing" History of Present Illness: 77 y o woman in NAD with a h/o CAD, DM2, asthma and HTN on altace for 8 years awoke from sleep around 2:30 am this morning of a sore throat and swelling of tongue. She came immediately to the ED b/c she was having difficulty swallowing and some trouble breathing due to obstruction caused by the swelling. She has never had a similar reaction ever before and she did not have any associated SOB, chest pain, itching, or nausea. She has not noticed any rashes, and has been afebrile. She says that she feels like it is swollen down in her esophagus as well. In the ED she was given 25mg benadryl IV, 125 mg solumedrol IV and pepcid 20 mg IV. This has helped the swelling some but her throat still hurts and it hurts to swallow. Nothing else was able to relieve the pain and nothing make it worse though she has not tried to drink any fluids because of trouble swallowing. She denies any recent travel, recent exposure to unusual plants or animals or other allergens. She has not started any new medications, has not used any new lotions or perfumes and has not eaten any unusual foods. Patient has not taken any of her oral medications today. Surgical History: s/p vaginal wall operation for prolapse 2006 s/p Cardiac stent in 1999 s/p hystarectomy in 1970s s/p kidney stone retrieval 1960s Medical History: +CAD w/ Left heart cath in 2005 showing 40% LAD, 50% small D2, 40% RCA and 30% large OM; 2006 TTE showing LVEF 60-65% with diastolic dysfunction, LVH, mild LA dilation +Hyperlipidemia +HTN +DM 2, last A1c 6.7 in 9/2005 +Asthma/COPD +GERD +h/o iron deficiency anemia Social History: Patient lives in _______ with daughter _____ (919) _______. -

Prolonged Bleeding Following Tongue Piercing: a Case Report and Review of Complications R
Clinical Section Prolonged Bleeding Following Tongue Piercing: a Case Report and Review of Complications R. Glenn Rosivack, DMD, MS Juei Yi Kao, DMD Dr. Rosivack is clinical associate professor, Department of Pediatric Dentistry, UMDNJ- New Jersey Dental School, Newark, NJ; Dr. Kao is in private practice, Danvers, Mass. Correspond with Dr. Rosivack at [email protected] Abstract The number of adolescents and young adults undergoing intraoral piercing is increasing in the United States. Numerous articles have documented complications following in- traoral piercing. This article presents a case of prolonged bleeding and reviews other documented sequelae following intraoral piercing. The article may serve as a guide to dental professionals whose patients seek advice regarding these procedures. (Pediatr Dent. 2003;25:154-156) KEYWORDS: BLEEDING, TONGUE PIERCING, ORAL Received April 8, 2002 Revision Accepted November 1, 2002 n increasingly popular form of body art involves also a possibility of aspiration. There was a reported case piercing various parts of the body. Piercing may be of pain, inflammation, and edema associated with place- performed on the eyebrows, lips, tongue, nares, na- ment of a metal barbell in the piercing site of the tongue.6 A 1 vel, nipples, and genitals. The most common intraoral site The concern of the possibility of keloid formation in sus- for piercing is the tongue.2 Dental professionals are increas- ceptible patients has also been raised. 8 Finally, it has been clinical section ingly being asked for guidance regarding this procedure by noted that the jewelry placed into the tongue can cause in- their teenage and young adult patients. -
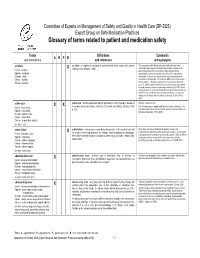
Glossary of Terms Related to Patient and Medication Safety
Committee of Experts on Management of Safety and Quality in Health Care (SP-SQS) Expert Group on Safe Medication Practices Glossary of terms related to patient and medication safety Terms Definitions Comments A R P B and translations and references and synonyms accident accident : an unplanned, unexpected, and undesired event, usually with adverse “For many years safety officials and public health authorities have Xconsequences (Senders, 1994). discouraged use of the word "accident" when it refers to injuries or the French : accident events that produce them. An accident is often understood to be Spanish : accidente unpredictable -a chance occurrence or an "act of God"- and therefore German : Unfall unavoidable. However, most injuries and their precipitating events are Italiano : incidente predictable and preventable. That is why the BMJ has decided to ban the Slovene : nesreča word accident. (…) Purging a common term from our lexicon will not be easy. "Accident" remains entrenched in lay and medical discourse and will no doubt continue to appear in manuscripts submitted to the BMJ. We are asking our editors to be vigilant in detecting and rejecting inappropriate use of the "A" word, and we trust that our readers will keep us on our toes by alerting us to instances when "accidents" slip through.” (Davis & Pless, 2001) active error X X active error : an error associated with the performance of the ‘front-line’ operator of Synonym : sharp-end error French : erreur active a complex system and whose effects are felt almost immediately. (Reason, 1990, This definition has been slightly modified by the Institute of Medicine : “an p.173) error that occurs at the level of the frontline operator and whose effects are Spanish : error activo felt almost immediately.” (Kohn, 2000) German : aktiver Fehler Italiano : errore attivo Slovene : neposredna napaka see also : error active failure active failures : actions or processes during the provision of direct patient care that Since failure is a term not defined in the glossary, its use is not X recommended. -

Section IX – Digestive System
Section IX – Digestive System The digestive system refers to the alimentary canal or gastrointestinal tract. It consists of organs and glands that break down food products to be used by the body as a source of energy through absorption of nutrients and to eliminate solid waste products. The GI tract begins at the mouth, where food is ingested and ends at the anus, where waste products are eliminated from the body. Medical Terms Combining Forms gingiv/o gums stomat/o, or/o mouth dent/o, odont/o teeth labi/o lip maxill/o jaw sial/o saliva, salivary glands abdomin/o abdomen gastr/o stomach lingu/o, gloss/o tongue myc/o fungus orth/o straight esophag/o esophagus carcin/o cancer rect/o rectum proct/o rectum and anus sigmoid/o sigmoid ile/o ileum col/o colon duoden/o duodenum enter/o intestine hepat/o liver chol/e, chol/o bile, gall cholecyst/o gall bladder pancreat/o pancreas cyst/o bladder an/o anus Suffixes -rrhea discharge, flow -orexia appetite -emesis vomiting -pepsia digestion -phagia swallowing, eating -lith stone Medical Terms Mouth (or/o, stomat/o) or/al, stomat/ic – pertaining to the mouth stomat/o/dynia – pain in the mouth sub/lingu/al – pertaining to under the tongue sub/maxill/ary – pertaining to under the jaw Salivary Glands (sial/o) sial/o/rrhea – excessive flow of saliva sial/o/aden/itis – inflammation of the salivary glands Teeth (odont/o, dent/o) dent/ist – specialist in the study of teeth orth/o/dont/ist – specialist in straight teeth gingiv/itis – inflammation of the gums Stomach (gastr/o) gastr/o/dynia – pain in the stomach -

Exercises to Strengthen the Tongue and Throat (Pharynx)
Page 1 of 1 Exercises to Strengthen the Tongue and Throat (Pharynx) These exercises help strengthen swallowing muscles. 6. Shaker: Improves the movement of the epiglottis and strengthens the opening of the esophagus. Also 1. Yawning: Helps upward movement of the larynx promotes upward movement of the larynx. (voice box) and the opening of the esophagus. Lie on your back, keeping your shoulders flat on the Open jaw as far as you can and hold for 10 seconds. ground. Raise your head far enough to be able to Rest for 10 seconds. Do 5 reps 2 times per day. see your toes and hold for 1 minute and then rest. 2. Effortful swallow: Improves movement of the Do 3 reps 3 times per day. tongue base and pharynx (throat). 7. Resistive tongue exercise: Improves tongue strength As you swallow, imagine you have a golf ball stuck and control of food and drink. in your throat. Squeeze as hard as you can with your Push tongue hard against roof of mouth. throat muscles. Do ___ reps ___ times per day. Push tongue hard against each cheek. 3. Mendelsohn: Promotes movement of the epiglottis. Push tongue hard against a tongue depressor Improves the function of the larynx and strength of or spoon. the esophageal opening. Hold for ___ seconds. Swallow and hold halfway through swallow (at Do ___ reps ___ times per day. highest point) for 1 to 2 seconds. Finish swallowing. Do ___ reps ___ times per day. 4. Tongue hold (Masako Maneuver): Helps strengthen tongue muscles needed for swallowing. Airway Swallow while holding your tongue tip 3/4 of an inch outside of your teeth. -

Nasal Breathing No Tongue Habits Lip Seal Corruption of the “Big 3” Is the Root Cause of Nearly All Malocclusions
Timothy Gross, DMD Part One In the Physiologic Orthodontics class at the Las Vegas Institute, we focus heavily on the “Big 3.” Orthodontic treatment and post treatment orthodontic stability necessitate management and correction of the “Big 3.” So, what is the “Big 3?” 1 2 3 Nasal Breathing No Tongue Habits Lip Seal Corruption of the “Big 3” is the root cause of nearly all malocclusions. That’s right. Class I, Class II, and Class III malocclusions, crowding, deficient midfaces, cross bites, impacted teeth, high palatal arches, deep bites, open bites (and the list goes on) can all be explained by Enlow in his textbook, “The Essentials of Facial Growth.” That textbook is paramount for understanding normal facial growth and development and should be required reading for every dentist and dental specialist. Malocclusions, so predominant in our society, cannot be explained by genetics alone. Our phenotype, i.e. our genome in addition to environmental influences, is the result of corruption of the “Big 3.” The inability to breathe nasally, habitual mouth straight and make the upper and lower teeth couple breathing, and/or tongue habits lead to altered nicely. Unfortunately, the result is a skeletal and facial growth patterns which in turn lead to physiologic malocclusion with straight teeth, at observable dental and orthognathic malocclusions. the expense of the airway, the temporomandibular Orthodontic treatment is utilized to correct these joints and facial aesthetics. malocclusions, but the underlying pathophysiology must be addressed in order to achieve an optimal A Case Study… Me physiologic occlusion, balanced facial growth and As a 48 year old male, I had multiple issues: a facial beauty. -

LZC502: Human Physiology Unit 1: Nutrition and Digestion
LZC502: Human Physiology Unit 1: Nutrition and Digestion Contents What is digestion Process of digestion Gastro intestinal tract or alimentary canal (Mouth, Pharynx, Esophagus, Stomach, Small and Large Intestine) Accessory digestive organs (Teeth, Tongue, Salivary gland, Liver, Gall bladder and Pancreas) What is Digestion ? Digestion is the mechanical and chemical breakdown of food substances into smaller components that are more easily absorbed into blood stream. Digestion is a form of catabolism that includes breakdown of large food molecules to smaller ones. Digestive system The organs involved in the breakdown of food are collectively called as digestive system. Digestive system composed of two groups of organs- Gastro intestinal tract or alimentary canal (Mouth, Pharynx, Esophagus, Stomach, Small and Large Intestine) Accessory digestive organs (Teeth, Tongue, Salivary gland, Liver, Gallbladder and Pancreas) Human digestive system Processes of Digestive system • Ingestion: this is the consumption of or taking in of nutrients. • Secretion: about 7 liter of water, acid, buffer and enzyme into the lumen. • Mixing and propulsion: contraction and relaxation of smooth muscle. • Digestion: Mechanical process: Breaking up food into smaller pieces. Chemical process: Breaking down food into molecules small enough to be absorbed into cells • Absorption: the transport or delivery of digested nutrients to body tissues. • Defecation: the elimination of food waste materials, bacteria and not absorbed digestive material from the body. Accessory digestive organs: Teeth Teeth are small whitish structures found in the jaws that are used to tear, scrape and chew food. Human have to dentition: deciduous, primary or milk teeth Dental formula: 2120 2120 permanent teeth Dental formula: 2123 2123 Accessory digestive organs: Tongue The tongue is an accessory digestive organ which along with the cheeks, keeps food between the upper and lower teeth until it's sufficiently masticated , or chewed. -

Normal Gastrointestinal Motility and Function Esophagus
Normal Gastrointestinal Motility and Function "Motility" is an unfamiliar word to many people; it is used primarily to describe the contraction of the muscles in the gastrointestinal tract. Because the gastrointestinal tract is a circular tube, when these muscles contract, they close off the tube or make the opening inside smaller - they squeeze. These muscles can contract in a synchronized way to move the food in one direction (usually downstream, but occasionally upstream for short distances); this is called peristalsis. If you looked at the intestine, you would see a ring of contraction that moves along pushing contents ahead of it. At other times, the muscles in adjacent parts of the gastrointestinal tract squeeze more or less independently of each other: this has the effect of mixing the contents but not moving them up or down. Both kinds of contraction patterns are called motility. The gastrointestinal tract is divided into four distinct parts: the esophagus, stomach, small intestine, and large intestine (colon). They are separated from each other by special muscles called sphincters which normally stay tightly closed and which regulate the movement of food and food residues from one part to another. Each part of the gastrointestinal tract has a unique function to perform in digestion, and as a result each part has a distinct type of motility and sensation. When motility or sensations are not appropriate for performing this function, they cause symptoms such as bloating, vomiting, constipation, or diarrhea which are associated with subjective sensations such as pain, bloating, fullness, and urgency to have a bowel movement.