Consensus Reclassification of Inherited
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Idiopathic Spiny Keratoderma: a Report of Two Cases and Literature Review
Idiopathic Spiny Keratoderma: A Report of Two Cases and Literature Review Jessica Schweitzer, DO,* Matthew Koehler, DO,** David Horowitz, DO*** *Intern, Largo Medical Center, Largo, FL **Dermatology Resident, Third Year, College Medical Center/Western University, Long Beach, CA ***Dermatology Residency Program Director, College Medical Center/Western University, Long Beach, CA Abstract Spiny keratoderma is a rare and likely underreported condition that presents with punctate hyperkeratotic growths localized to the palms and soles. We present two cases of clinically diagnosed spiny keratoderma. Although the lesions were asymptomatic, patients are at risk of an underlying internal malignancy with this condition, so diagnosis is crucial. Neither men were seeking treatment for the lesions when they were discovered, suggesting that this condition may be much more common than reported. Patients with histories of manual labor, increased UV exposure, and non-melanoma skin cancer (NMSC) may also be at higher risk for developing spiny keratoderma.1 The epidemiology, histopathologic features, differential diagnosis, and current treatments for spiny keratoderma are reviewed. Introduction Case 2 enthusiast for his entire life, spending significant Spiny keratoderma is a rare palmoplantar A 67-year-old Caucasian male presented with a time using his hands to maintain and fire his keratoderma that presents with keratotic, pinpoint one-year history of insidiously growing, pinpoint weapons and many hours outside without sun papules on the palms and soles. There are both hyperkeratotic papules projecting from his palms protection. The patient was referred back to his hereditary and acquired forms. When found, bilaterally (Figures 4-5). He presented to the clinic primary care physician for internal evaluation. -

PGD: a Celebration of 20 Years
PGD: A Celebration of 20 years: What is Reality and What is Not? Roma June 30, 2010 Mark Hughes, M.D., Ph.D . Professor of Genetics, Internal Medicine, Pathology Director, Genesis Genetics Institute Director, State of Michigan Genomic Technology Center Reality – (Three obvious ones) PGD • Has led to the birth of thousands of healthy children to very desperate, genetically at-risk couples. • Remains at the very limit of medical diagnostic testing • The technology continues to improve - – but it is not reality to think PGD will ever have a 0% false positive or false negative rate Reality: We still do not know What is best to biopsy, and when? Polar Body Blastomere Trophoectoderm Variation in Biopsy Skill Clinic Biopsies +HCG / ET 1 314 17% 2 427 26% 3 181 12% 4 712 31% Reality: We all are controversial • PGD has raised international controversy – How is it bioethically different from Prenatal Testing? – Who should control the use of these technologies? – Should there be government PGD testing standards? • What is the difference between a Disease and a Trait - and who decides? PGD Disorders (A, B, C) • ACHONDROPLASIA (FGFR) • BARTH DILIATED CARDIOMYOPATHY • ACTIN-NEMALIN MYOPATHY (ACTA) • BETA THALASSEMIA (HBB) • ADRENOLEUKODYSTROPHY (ABCD) • BLOOM SYNDROME • AGAMMAGLOBULINEMIA-BRUTON (TYKNS) • BREAST CANCER (BRCA1 & 2) • ALAGILLE SYNDROME (JAG) • CACH-ATAXIA (EIFB) • ALDOLASE A, FRUCTOSE-BISPHOSPHATE • CADASIL (NOTCH) • ALPHA THALASSEMIA (HBA) • CANAVAN DISEASE (ASPA) • ALPHA-ANTITRYPSIN (AAT) • CARNITINE-ACYLCARN TRANSLOCASE • ALPORT SYNDROME -

Ectodermal Dysplasias: a New Clinical-Genetic Classification
J Med Genet 2001;38:579–585 579 Ectodermal dysplasias: a new clinical-genetic J Med Genet: first published as 10.1136/jmg.38.9.579 on 1 September 2001. Downloaded from classification Manuela Priolo, Carmelo Laganà Abstract many case reports and personal communica- The ectodermal dysplasias (EDs) are a tions in their listing of EDs, as well as large and complex nosological group of conditions traditionally classified under other diseases, first described by Thurnam in headings, for example dyskeratosis congenita11 1848. In the last 10 years more than 170 and keratitis-ichthyosis-deafness (KID) syn- diVerent pathological clinical conditions drome12 (poikiloderma and immune defect have been recognised and defined as EDs, diseases and erythrokeratodermas, respec- all sharing in common anomalies of the tively). Further, they did not appear to hair, teeth, nails, and sweat glands. Many consider variability of expression and may are associated with anomalies in other have reported, as distinct diseases, conditions organs and systems and, in some condi- that reflect variable expression of the same tions, with mental retardation. pathological entity. Moreover, they included The anomalies aVecting the epidermis pathological conditions which, in our opinion, and epidermal appendages are extremely do not strictly fulfil the diagnostic criteria for variable and clinical overlap is present EDs, such as conditions with secondary among the majority of EDs. Most EDs are involvement of epidermal derivatives rather defined by particular clinical signs (for than a primary defect. We abandoned the 1-2- example, eyelid adhesion in AEC syn- 3-4 designation of EDs, because we believe drome, ectrodactyly in EEC). -

Associated Palmoplantar Keratoderma
DR ABIGAIL ZIEMAN (Orcid ID : 0000-0001-8236-207X) Article type : Review Article Pathophysiology of pachyonychia congenita-associated palmoplantar keratoderma: New insight into skin epithelial homeostasis and avenues for treatment Authors: A. G. Zieman1 and P. A. Coulombe1,2 # Affiliations: 1Department of Cell and Developmental Biology, University of Michigan Medical School, Ann Arbor, MI 48109, USA; 2Department of Dermatology, University of Michigan Medical School, Ann Arbor, MI 48109, USA #Corresponding author: Pierre A. Coulombe, PhD, 3071 Biomedical Sciences Research Building, 109 Zina Pitcher Place, Ann Arbor, MI 48109, USA. Tel: 734-615-7509. Email: [email protected]. Funding Sources: These studies were supported by grant AR044232 issued to P.A.C. from the National Institute of Arthritis, Musculoskeletal and Skin Disease (NIAMS). A.G.Z. received support from grant T32 CA009110 from the National Cancer Institute. Author Manuscript This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/BJD.18033 This article is protected by copyright. All rights reserved Conflict of interest disclosures: None declared. Bulleted statements: What’s already known about this topic? Pachyonychia congenita is a rare genodermatosis caused by mutations in KRT6A, KRT6B, KRT6C, KRT16, KRT17, which are normally expressed in skin appendages and induced following injury. Individuals with PC present with multiple clinical symptoms that usually include thickened and dystrophic nails, palmoplantar keratoderma (PPK), glandular cysts, and oral leukokeratosis. -

Palmoplantar Keratoderma with Progressive Gingivitis and Recurrent Pyodermas
Palmoplantar Keratoderma With Progressive Gingivitis and Recurrent Pyodermas Tyler A. Moss, DO; Anne P. Spillane, MD; Sam F. Almquist, MD; Patrick E. McCleskey, MD; Oliver J. Wisco, DO Practice Points Papillon-Lefèvre syndrome (PLS) is an autosomal-recessive inherited transgredient palmoplantar kerato- derma (PPK) that is associated with gingivitis and recurrent pyodermas. The symptoms associated with PLS are thought to be due to cathepsin C gene, CTSC, mutations. CTSC is expressed in epithelial regions commonly affected by PLS and also plays a role in the activation of immune and inflammatory responses. Papillon-Lefèvre syndrome must be differentiated from other conditions causing PPK, such as Haim-Munk syndrome, Greither syndrome, mal de Meleda, Clouston syndrome, Vohwinkel syndrome, and Olmsted syndrome. Treatment of PLS includesCUTIS keratolytics such as urea and/or salicylic acid comb ined with oral retinoids. Active gingivitis may be treated with combined use of amoxicillin and metronidazole. Papillon-Lefèvre syndrome (PLS) is a rare inher- Case Report ited palmoplantar keratoderma (PPK) that is asso- A 30-year-old woman presented to the dermatology ciated with progressive gingivitis and recurrent clinic with erythematous hyperkeratotic plaques on pyodermas.Do We present a caseNot exhibiting classic the palmsCopy and soles. The plaques extended onto features of this autosomal-recessive condition the dorsal aspects of the fingers, toes, hands, and and review the current understanding of its patho- feet (Figures 1 and 2). The patient had psoriasiform physiology, diagnosis, and treatment. Addition- plaques on the extensor surfaces of the knees and ally, a review of pertinent transgredient PPKs is elbows (Figure 3) along with a history of slow- undertaken, with key and distinguishing features progressing gingivitis and periodontal disease that of each syndrome highlighted. -

Revertant Mosaicism in a Human Skin Fragility Disorder Results from Slipped Mispairing and Mitotic Recombination
Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination Dimitra Kiritsi, … , Leena Bruckner-Tuderman, Cristina Has J Clin Invest. 2012;122(5):1742-1746. https://doi.org/10.1172/JCI61976. Brief Report Dermatology Spontaneous gene repair, also called revertant mosaicism, has been documented in several genetic disorders involving organs that undergo self-regeneration, including the skin. Genetic reversion may occur through different mechanisms, and in a single individual, the mutation can be repaired in various ways. Here we describe a disseminated pattern of revertant mosaicism observed in 6 patients with Kindler syndrome (KS), a genodermatosis caused by loss of kindlin-1 (encoded by FERMT1) and clinically characterized by patchy skin pigmentation and atrophy. All patients presented duplication mutations (c.456dupA and c.676dupC) in FERMT1, and slipped mispairing in direct nucleotide repeats was identified as the reversion mechanism in all investigated revertant skin spots. The sequence around the mutations demonstrated high propensity to mutations, favoring both microinsertions and microdeletions. Additionally, in some revertant patches, mitotic recombination generated areas with homozygous normal keratinocytes. Restoration of kindlin-1 expression led to clinically and structurally normal skin. Since loss of kindlin-1 severely impairs keratinocyte proliferation, we predict that revertant cells have a selective advantage that allows their clonal expansion and, consequently, the improvement of the skin condition. Find the latest version: https://jci.me/61976/pdf Brief report Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination Dimitra Kiritsi,1 Yinghong He,1 Anna M.G. Pasmooij,2 Meltem Onder,3 Rudolf Happle,1 Marcel F. -

Erythrokeratodermia Variabilis Et Progressiva Allelic to Oculo-Dento
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector COMMENTARY See related article on pg 1540 translocated into the plasma membrane. Once expressed on the cell surface, the hemichannel docks with a connexon of an adjacent cell to form a channel that Erythrokeratodermia Variabilis et is termed gap junction. Connexons can form either homotypic (docking of two Progressiva Allelic to Oculo-Dento- identical connexons), heterotypic (docking of two dissimilar homomeric Digital Dysplasia connexons), or heteromeric (docking of two heteromeric connexons) channels Sabine Duchatelet1,2 and Alain Hovnanian1,2,3 (Mese et al., 2007). These diverse Erythrokeratodermia variabilis et progressiva (EKVP) is a genodermatosis with combinations of connexins create clinical and genetic heterogeneity, most often transmitted in an autosomal different types of channels, each having dominant manner, caused by mutations in GJB3 and GJB4 genes encoding unique properties (ionic conductance, connexins (Cx)31 and 30.3, respectively. In this issue, Boyden et al. (2015) report permeability, sensitivity to voltage, or for the first time de novo dominant mutations in GJA1 encoding the ubiquitous pH). Of note, several connexins may also Cx43 in patients with EKVP. These results expand the genetic heterogeneity of form functional nonjunctional hemi- EKVP and the human disease phenotypes associated with GJA1 mutations. They channels, although their physiological disclose that EKVP is allelic to oculo-dento-digital dysplasia, a rare syndrome relevance remains uncertain (Pfenniger previously known to be caused by dominant GJA1 mutations. et al., 2010). Mutations in 11 connexin genes cause a variety of genetic dis- Journal of Investigative Dermatology (2015) 135, 1475–1478. -

A Review of the Evidence for and Against a Role for Mast Cells in Cutaneous Scarring and Fibrosis
International Journal of Molecular Sciences Review A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis Traci A. Wilgus 1,*, Sara Ud-Din 2 and Ardeshir Bayat 2,3 1 Department of Pathology, Ohio State University, Columbus, OH 43210, USA 2 Centre for Dermatology Research, NIHR Manchester Biomedical Research Centre, Plastic and Reconstructive Surgery Research, University of Manchester, Manchester M13 9PT, UK; [email protected] (S.U.-D.); [email protected] (A.B.) 3 MRC-SA Wound Healing Unit, Division of Dermatology, University of Cape Town, Observatory, Cape Town 7945, South Africa * Correspondence: [email protected]; Tel.: +1-614-366-8526 Received: 1 October 2020; Accepted: 12 December 2020; Published: 18 December 2020 Abstract: Scars are generated in mature skin as a result of the normal repair process, but the replacement of normal tissue with scar tissue can lead to biomechanical and functional deficiencies in the skin as well as psychological and social issues for patients that negatively affect quality of life. Abnormal scars, such as hypertrophic scars and keloids, and cutaneous fibrosis that develops in diseases such as systemic sclerosis and graft-versus-host disease can be even more challenging for patients. There is a large body of literature suggesting that inflammation promotes the deposition of scar tissue by fibroblasts. Mast cells represent one inflammatory cell type in particular that has been implicated in skin scarring and fibrosis. Most published studies in this area support a pro-fibrotic role for mast cells in the skin, as many mast cell-derived mediators stimulate fibroblast activity and studies generally indicate higher numbers of mast cells and/or mast cell activation in scars and fibrotic skin. -

Foot Pain in Scleroderma
Foot Pain in Scleroderma Dr Begonya Alcacer-Pitarch LMBRU Postdoctoral Research Fellow 20th Anniversary Scleroderma Family Day 16th May 2015 Leeds Institute of Rheumatic and Musculoskeletal Medicine Presentation Content n Introduction n Different types of foot pain n Factors contributing to foot pain n Impact of foot pain on Quality of Life (QoL) Leeds Institute of Rheumatic and Musculoskeletal Medicine Scleroderma n Clinical features of scleroderma – Microvascular (small vessel) and macrovascular (large vessel) damage – Fibrosis of the skin and internal organs – Dysfunction of the immune system n Unknown aetiology n Female to male ratio 4.6 : 1 n The prevalence of SSc in the UK is 8.21 per 100 000 Leeds Institute of Rheumatic and Musculoskeletal Medicine Foot Involvement in SSc n Clinically 90% of SSc patients have foot involvement n It typically has a later involvement than hands n Foot involvement is less frequent than hand involvement, but is potentially disabling Leeds Institute of Rheumatic and Musculoskeletal Medicine Different Types of Foot Pain Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Microvascular disease (small vessel) n Intermittent pain – Raynaud’s (spasm) • Cold • Throb • Numb • Tingle • Pain n Constant pain – Vessel center narrows • Distal pain (toes) • Gradually increasing pain • Intolerable pain when necrosis is present Leeds Institute of Rheumatic and Musculoskeletal Medicine Ischaemic Pain (vascular) Macrovascular disease (large vessels) n Intermittent and constant pain – Peripheral Arterial Disease • Intermittent claudication – Muscle pain (ache, cramp) during walking • Aching or burning pain • Night and rest pain • Cramps Leeds Institute of Rheumatic and Musculoskeletal Medicine Ulcer Pain n Ulcer development – Constant pain n Infected ulcer – Unexpected/ excess pain or tenderness Leeds Institute of Rheumatic and Musculoskeletal Medicine Neuropathic Pain n Nerve damage is not always obvious. -

Emaciation, Congestive Heart Failure, and Systemic Amyloidosis In
Case Report Emaciation, Congestive Heart Failure, and Systemic Amyloidosis in Severe Recessive Dystrophic Epidermolysis Bullosa: Possible Internal Complications Due to Skin-Derived Inflammatory Cytokines Derived from the Injured Skin Yoshiaki Matsushima y , Kento Mizutani y, Hiroyuki Goto y, Takehisa Nakanishi, Makoto Kondo, Koji Habe, Kenichi Isoda, Hitoshi Mizutani and Keiichi Yamanaka * Department of Dermatology, Mie University Graduate School of Medicine, Tsu, Mie 514-8507, Japan; [email protected] (Y.M.); [email protected] (K.M.); [email protected] (H.G.); [email protected] (T.N.); [email protected] (M.K.); [email protected] (K.H.); [email protected] (K.I.); [email protected] (H.M.) * Correspondence: [email protected]; Tel.: +81-59-231-5025; Fax: +81-59-231-5206 These authors contributed equally to this work. y Received: 2 August 2020; Accepted: 7 September 2020; Published: 14 September 2020 Abstract: Inherited epidermolysis bullosa (EB) is a rare genetic skin disorder characterized by epithelial tissue fragility. Recessive dystrophic epidermolysis bullosa (RDEB) is the most severe form, characterized by the presence of blisters, erosion, and ulcer formation, leading to scarring and contraction of the limbs. RDEB is also associated with extra-cutaneous complications, including emaciation, congestive heart failure, and systemic amyloidosis. The main cause of these clinical complications is unknown; however, we hypothesized that they are caused by elevated circulating inflammatory cytokines overproduced by injured keratinocytes. We addressed this phenomenon using keratin-14 driven, caspase-1 overexpressing, transgenic (KCASP1Tg) mice in which injured keratinocytes release high levels of IL-1α and β. -

WES Gene Package Multiple Congenital Anomalie.Xlsx
Whole Exome Sequencing Gene package Multiple congenital anomalie, version 5, 1‐2‐2018 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 607922 101 100 100 99 [Blood group, P1Pk system, p phenotype], 111400 NOR polyagglutination syndrome, 111400 AAAS Achalasia‐addisonianism‐alacrimia syndrome, 231550 605378 73 100 100 100 AAGAB Keratoderma, palmoplantar, -
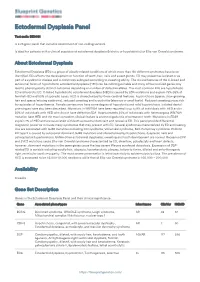
Blueprint Genetics Ectodermal Dysplasia Panel
Ectodermal Dysplasia Panel Test code: DE0401 Is a 25 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of ectodermal dysplasia (hidrotic or hypohidrotic) or Ellis-van Creveld syndrome. About Ectodermal Dysplasia Ectodermal Dysplasia (ED) is a group of closely related conditions of which more than 150 different syndromes have been identified. EDs affects the development or function of teeth, hair, nails and sweat glands. ED may present as isolated or as part of a syndromic disease and is commonly subtyped according to sweating ability. The clinical features of the X-linked and autosomal forms of hypohidrotic ectodermal dysplasia (HED) can be indistinguishable and many of the involved genes may lead to phenotypically distinct outcomes depending on number of defective alleles. The most common EDs are hypohidrotic ED and hydrotic ED. X-linked hypohidrotic ectodermal dysplasia (HED) is caused by EDA mutations and explain 75%-95% of familial HED and 50% of sporadic cases. HED is characterized by three cardinal features: hypotrichosis (sparse, slow-growing hair and sparse/missing eyebrows), reduced sweating and hypodontia (absence or small teeth). Reduced sweating poses risk for episodes of hyperthermia. Female carriers may have some degree of hypodontia and mild hypotrichosis. Isolated dental phenotypes have also been described. Mutations in WNT10A have been reported in up to 9% of individuals with HED and in 25% of individuals with HED who do not have defective EDA. Approximately 50% of individuals with heterozygous WNT10A mutation have HED and the most consistent clinical feature is severe oligodontia of permanent teeth.