Cervical Necrotic Lymphadenopathy: a Diagnostic Tree Analysis Model Based on CT and Clinical Findings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Isolated Cervical Lymph Node Sarcoidosis Presenting in an Asymptomatic Neck Mass: a Case Report
http://dx.doi.org/10.4046/trd.2013.75.3.116 CASE REPORT ISSN: 1738-3536(Print)/2005-6184(Online) • Tuberc Respir Dis 2013;75:116-119 Isolated Cervical Lymph Node Sarcoidosis Presenting in an Asymptomatic Neck Mass: A Case Report Yong Shik Kwon, M.D.1, Hye In Jung, M.D.1, Hyun Jung Kim, M.D.1, Jin Wook Lee, M.D.1, Won-Il Choi, M.D., Ph.D.1, Jin Young Kim, M.D.2, Byung Hak Rho, M.D., Ph.D.2, Hye Won Lee, M.D.3 and Kun Young Kwon, M.D., Ph.D.3 Departments of 1Internal Medicine, 2Radiology, and 3Pathology, Keimyung University Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea Sarcoidosis, a systemic granulomatous disease of unknown etiology. The presentation of sarcoidal granuloma in neck nodes without typical manifestations of systemic sarcoidosis is difficult to diagnose. We describe the case of a 37-year-old woman with an increasing mass on the right side of neck. The excisional biopsy from the neck mass showed noncaseating epithelioid cell granuloma of the lymph nodes. No evidence of mycobacterial or fungal infection was noted. Thoracic evaluations did not show enlargement of mediastinal lymph nodes or parenchymal abnormalities. Immunohistochemistry showed abundant expression of tumor necrosis factor-α in the granuloma. However, transforming growth factor-β was not expressed, although interleukin-1β was focally expressed. These immunohistochemical findings supported characterization of the granuloma and the diagnosis of sarcoidosis. Sarcoidosis can present with cervical lymph node enlargement without mediastinal or lung abnormality. Immunohistochemistry may support the diagnosis of sarcoidosis and characterization of granuloma. -
Managing Tooth Pain in General Practice
Singapore Med J 2019; 60(5): 224-228 Practice Integration & Lifelong Learning https://doi.org/10.11622/smedj.2019044 CMEARTICLE Managing tooth pain in general practice Sky Wei Chee Koh1, MBChB, Chun Fai Li2, BDS, John Ser Pheng Loh2, MDS, MBBS, Mun Loke Wong2, BDS, MSc, Victor Weng Keong Loh1, MCFP, MHPE Mr Tan, a 48-year-old banker with no significant past medical history, visited your clinic with the complaint of severe right-sided tooth pain that radiated to the right temporal region of the head. The pain was excruciating and had affected his concentration at work. The over- the-counter paracetamol he had taken did not seem to relieve the pain and Mr Tan felt that it could not just be a simple toothache. He asked you to prescribe some antibiotics to treat what he believed was a dental infection. WHAT IS TOOTH PAIN? infection.(6) This bidirectional relationship underscores the pivotal Tooth pain, which is often known as toothache, refers to the role that primary care physicians play in the prompt diagnosis, symptom of pain arising from the tooth (or teeth). investigation and management of patients with oral conditions. HOW COMMON IS THIS IN MY WHAT CAN I DO IN MY PRACTICE? PRACTICE? Clinical history and examination Dental caries (Fig. 1) is a common dental condition. Globally, up Many oral conditions may mimic tooth pain and it is important to to 35% of people have untreated dental caries,(1) and an estimated delineate the different causes with history-taking and examination. 32.4% of the Singapore population will experience pain from We suggest the following: symptomatic dental caries in their lifetime.(2) Locally, oral disease • Identify the source of pain by taking a comprehensive pain is ranked 16th in terms of years lost to disability and has been history. -

An Approach to Cervical Lymphadenopathy in Children
Singapore Med J 2020; 61(11): 569-577 Practice Integration & Lifelong Learning https://doi.org/10.11622/smedj.2020151 CMEARTICLE An approach to cervical lymphadenopathy in children Serena Su Ying Chang1, MMed, MRCPCH, Mengfei Xiong2, MBBS, Choon How How3,4, MMed, FCFP, Dawn Meijuan Lee1, MBBS, MRCPCH Mrs Tan took her daughter Emma, a well-thrived three-year-old girl, to the family clinic for two days of fever, sore throat and rhinorrhoea. Apart from slight decreased appetite, Mrs Tan reported that Emma’s activity level and behaviour were not affected. During the examination, Emma was interactive, her nose was congested with clear rhinorrhoea, and there was pharyngeal injection without exudates. You discovered bilateral non-tender, mobile cervical lymph nodes that were up to 1.5 cm in size. Mrs Tan was uncertain if they were present prior to her current illness. There was no organomegaly or other lymphadenopathy. You treated Emma for viral respiratory tract illness and made plans to review her in two weeks for her cervical lymphadenopathy. WHAT IS LYMPHADENOPATHY? masses in children can be classified into congenital or acquired Lymphadenopathy is defined as the presence of one or more causes. Congenital lesions are usually painless and may be identified lymph nodes of more than 1 cm in diameter, with or without an at or shortly after birth. They may also present with chronic drainage abnormality in character.(1) In children, it represents the majority or recurrent episodes of swelling, which may only be obvious in later of causes of neck masses, which are abnormal palpable lumps life or after a secondary infection. -

Infectious Mononucleosis Mimicking Lymphoma: Distinguishing Morphological and Immunophenotypic Features
Modern Pathology (2012) 25, 1149–1159 & 2012 USCAP, Inc. All rights reserved 0893-3952/12 $32.00 1149 Infectious mononucleosis mimicking lymphoma: distinguishing morphological and immunophenotypic features Abner Louissaint Jr, Judith A Ferry, Chad P Soupir, Robert P Hasserjian, Nancy L Harris and Lawrence R Zukerberg The James Homer Wright Pathology Laboratories, Massachusetts General Hospital, Boston, MA, USA The diagnosis of infectious mononucleosis (acute Epstein–Barr virus (EBV) infection) is usually made on the basis of clinical and laboratory findings. However, an atypical clinical presentation occasionally results in a lymph node or tonsillar biopsy. The morphological features of EBV-infected lymphoid tissue can easily mimic lymphoma. Furthermore, the immunophenotype of the immunoblasts has not been well characterized. To assess the morphological spectrum of acute EBV infection and the utility of immunohistochemistry in diagnosing difficult cases that resemble lymphoma, we reviewed 18 cases of acute EBV infection submitted in consultation to our institution with an initial diagnosis of/or suspicion for lymphoma. Patients included nine male and nine female individuals with a median age of 18 years (range 9–69). Biopsies were obtained from lymph nodes (3/18) or Waldeyer’s ring (15/18). Infectious mononucleosis was confirmed by monospot or serological assays in 72% of cases (13/18). All cases featured architectural distortion by a polymorphous infiltrate with an immunoblastic proliferation, sometimes forming sheets. Reed–Sternberg-like cells were present in 8/18 (44%) of the cases. Infiltrates were often accompanied by necrosis (10/18) and mucosal ulceration (6/15). The majority of immunoblasts in all cases were CD20 þ B cells with a post-germinal center immunophenotype (strongly positive for MUM1/IRF4 (18/18), CD10À (18/18 negative) and BCL-6À (16/18 negative; 2/18 faint BCL-6 expression in o10% of immunoblasts)). -

Original Article Prevalence of Metastatic Neck Nodes Md
Bangladesh J Otorhinolaryngol 2019; 25(2): 102-107 Original Article Prevalence of Metastatic Neck Nodes Md. Momin Uddin1, Samia Quadir2, Sabiha Quadir3, Kazi Shameemus Salam4, Debabrota Roy5, Belayat Hossain Siddiquee6 Abstract: Background: Head and neck cancers include cancers of the lips, mouth, nasal cavity, paranasal sinuses, pharynx and larynx. Most of these cancers are squamous cell carcinomas (SCCs). The presence of metastatic cervical lymphadenopathy is of particular importance as with every single nodal metastasis, survival of the patient is reduced by one half. Objective: To see the prevalence of metastatic neck node. Methods: The prospective cross-sectional clinical study was carried out in the Department of ENT and Head Neck Surgery, Combined Military Hospital, Dhaka during March’2018 to March, 2019. All 100 patients were included in this study and were treated at the Department of Otolaryngology of Combined Military Hospital, Dhaka Results: Total 26 cases were found parotid among them 8(30.8%) in metastatic neck node and 18(69.2%) in without metastatic neck node. Total 10 cases were found paranasal sinuses among them 1(10.0%) in metastatic neck node and 9(90.0%) in without metastatic neck node. Which were statistically significant (p<0.05) between two groups. Conclusion: In this study observed that majority of metastatic neck node were found pyriform fossa, supraglottic larynx, base of tongue which were 68.2%, 68%, 77.8% respectively. In oral cavity and parotid site also found 48.1% and 30.8% metastatic neck node. 1. Classified ENT Specialist & Head-Neck Introduction: Surgeon, CMH, Jashore Cantonment. Head and neck cancers include cancers of 2. -
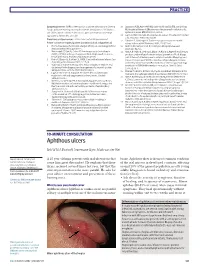
Aphthous Ulcers
PRACTICE Competing interests: RMB has been paid as an adviser to Numico and Schering 10 Luccassen PLBJ, Assendelft WJJ, Gubbels JW, van Eijk JTM, van Geldrop Plough and has received sponsorship from Nestle, Mead Johnson, SHS, Nutricia, WJ, Knuistingh Neven A. Effectiveness of treatments for infantile colic: and SMA to attend conferences. He has also given presentations at meetings systematic review. BMJ 1998;316:1563-9. sponsored by Nestle, SHS, and SMA. 11 Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics 2000;106:184-90. Provenance and peer review: Commissioned; externally peer reviewed. 12 Salvatore S, Vandenplas Y. Gastroesophageal reflux and cow milk Patient consent not required (patient anonymised, dead, or hypothetical). allergy: is there a link? Pediatrics 2002;110:972-84. 1 Host A. Frequency of cow’s milk allergy in childhood. Ann Allergy Asthma 13 Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol Immunol 2002;89(6 suppl 1):33-7. 2006;117:S470-5. 2 Macdougall CF, Cant AJ, Colver AF. How dangerous is food allergy in 14 Høst A, Koletzko B, Dreborg S, Muraro A, Wahn U, Aggett P, et al. Dietary childhood? The incidence of severe and fatal allergic reactions across products used in infants for treatment and prevention of food allergy. the UK and Ireland. Arch Dis Child 2002;86:236-9. Joint statement of the European Society for Paediatric Allergology and 3 Heine R, Elsayed S, Hosking CS, Hill DJ. Cow’s milk allergy in infancy. Curr Clinical Immunology (ESPACI) committee on hypoallergenic formulas Opin Allergy Clin Immunol 2002;2:217-25. -

Case Presentation - Atypical Mycobacterial Mar 2016; Published Date: 21 Mar 2016
ISSN 2470-0991 SciForschenOpen HUB for Scientific Research Journal of Surgery: Open Access Case Report Volume: 2.4 Open Access Received date: 15 Feb 2016; Accepted date: 16 Case Presentation - Atypical Mycobacterial Mar 2016; Published date: 21 Mar 2016. Cervical Lymphadenitis Citation: Patel N, Ranganathan B, Faris C, Kumar N (2016) Case Presentation - Atypical Mycobacterial Neelam Patel1*, Ranganathan B2, Faris C3 and Kumar N2 Cervical Lymphadenitis. J Surg Open Access 2(4): doi http://dx.doi.org/10.16966/2470-0991.118 1Foundation Year 2 Doctor, Wrightington, Wigan and Leigh Trust, Wigan Lane, Wigan, Lancashire, UK 2Consultant ENT Surgeon, Wrightington, Wigan and Leigh Trust, Wigan Lane, Wigan, Lancashire, UK Copyright: © 2016 Patel N, et al. This is an 3Consultant Microbiologist, Wrightington, Wigan and Leigh Trust, Wigan Lane, Wigan, Lancashire, UK open-access article distributed under the terms of the Creative Commons Attribution License, *Corresponding author: Neelam Patel, Foundation Year 2 Doctor, Wrightington, Wigan and Leigh which permits unrestricted use, distribution, and Trust, Wigan Lane, Wigan, Lancashire, UK, Tel: 07859904753; E-mail: [email protected] reproduction in any medium, provided the original author and source are credited. Abstract Neck lumps are a very common presentation to hospital in pediatric patients. Lymphadenitis secondary to atypical mycobacterium infections are rare but recently there has been an increase in prevalence in the UK. The treatment of such cases is controversial; the options of medical, surgical or combination of both are practiced, with variations, in different centres around the world. We report a case of a 4 year old girl who presented with a short history of a neck lump due to Mycobacterium Avium Complex (MAC) infection which was surgically excised, with good post-op and aesthetic outcome. -

Lymphadenopathy & Cervical Lymphadenitis Guidelines
LYMPHADENOPATHY & CERVICAL LYMPHADENITIS GUIDELINES Compiled by: Dr Rachel Panniker Dr Muhammad Yaqub In Consultation with: Dr Alison Groves Ratified by: Paediatric Guidelines Group Date Ratified: July 2019 Date Reviewed: July 2019 Next Review Date: June 2023 Target Audience: Doctors, nurses and support staff working in Paediatrics Impact Assessment Carried Out By: Comment on this document to: Dr Muhammad Yaqub (Consultant Paediatrician) First Ratified Latest Reviewed Version Number: Page 1 of 8 July 2019 July 2019 1 Lymphadenopathy "Lymphadenopathy" refers to enlargement of the lymph nodes. Lymphadenitis (see page 8) refers to enlarged, tender and inflamed lymph nodes. Lymphadenopathy in children is very common. 90% of children between the ages of 4 and 8 have palpable cervical lymph nodes. Lymphadenopathy most commonly represents a transient response of lymphatic tissue hyperplasia to a local benign inflammatory process. However it can also represent other more significant pathology including a neoplastic process. Causes: Viral infections Malignancies a. Upper Respiratory Tract 1. Hodgkin Lymphoma Infection Viruses 2. Non-Hodgkin lymphoma i. Rhinovirus 3. Neuroblastoma ii. Adenovirus 4. Leukaemia iii. Influenza virus 5. Rhabdomyosarcoma iv. Parainfluenza virus 6. Metastatic disease v. Respiratory synctial virus Parasitic b. Epstein Bar Virus Toxoplasmosis c. Cytomegalovirus d. Togavirus e. Varicella-zoster virus Dermatological f. Herpes Simplex virus 1. Eczema g. Paramyxovirus 2. Tinea Capitis h. Coxackievirus A and B 3. Tinea Corporis (ringworm) i. Echovirus j. Enterovirus k. Human Herpesvirus-6 l. Human Immunodeficiency Virus Bacterial Infections Miscellaneous 1. GAS (Group A Streptococcus) 1. Kawasaki disease 2. Group B Streptococcus 2. Drugs 3. Staphylococcus Aureus i) Phenytoin 4. Anaerobic bacteria (Dental Caries) ii) Isoniazid 5. -

Study of Relationship Between Maxillofacial Lesions and Cervical Lymphadenopathy
Study of relationship between maxillofacial lesions and cervical lymphadenopathy using MR imaging (MRI を用いた顎顔面疾患と頸部リンパ節腫脹の関連性) 日本大学大学院松戸歯学研究科 放射線学 村岡 宏隆 (指導: 金田 隆 教授) 1 本論文は、 1) Parotid lymphadenopathy is associated with joint effusion in nonneoplastic temporomandibular disorders Journal of Oral Maxillofacial Surgery (In press) 2) Lymphadenopathy of the Maxillofacial Area Caused by Periodontitis Journal of Hard Tissue Biology (第 26 巻 2 号 平成 29 年 4 月発行予定) をまとめたものである。 2 1. Abstract 2. Introduction 3. Material and Methods 3- 1. Parotid lymphadenopathy is associated with joint effusion in nonneoplastic temporomandibular disorders 3- 2. Lymphadenopathy of the maxillofacial area caused by periodontitis 4. Result 4- 1. Parotid lymphadenopathy is associated with joint effusion in nonneoplastic temporomandibular disorders 4- 2. Lymphadenopathy of the maxillofacial area caused by periodontitis 5. Discussion 5- 1. Parotid lymphadenopathy is associated with joint effusion in nonneoplastic temporomandibular disorders 5- 2. Lymphadenopathy of the maxillofacial area caused by periodontitis 6. Conclusions 7. References 8. Figure and legends 3 1, Abstract Purposes Joint effusion, a pathological accumulation of synovial fluid caused by inflammation, is easily identified on magnetic resonance imaging (MRI), and there have been many studies of its relationship to joint pain, disk displacement, and abnormal marrow signal. Periodontitis is an inflammatory disease of the supporting tissues of the teeth caused by specific microorganisms, resulting in progressive destruction of the periodontal ligament and alveolar bone with increased probing depth formation, recession, or both. Lymphadenopathy often occurs in the setting of inflammation with or without infection. There has been little attention devoted to the relationship between cervical lymphadenopathy and these maxillofacial lesions. -

Bilateral Hilar Lymphadenopathy with Infectious Mononucleosis
Case Report International Journal of Internal and Emergency Medicine Published: 16 May, 2018 Bilateral Hilar Lymphadenopathy with Infectious Mononucleosis Sangwoo Leem1, Rudaina Banihani1-4, John Wong1-3, Savithiri Ratnapalan1-3, Peter Wong1-3,5 and Yousef Etoom1-3,6* 1Department of Medicine, University of Toronto, Canada 2Division of Pediatric Medicine, University of Toronto, Canada 3Department of Pediatrics, The Hospital for Sick Children, Canada 4Department of Newborn and Developmental Pediatrics, Sunnybrook Health Science Centre, Canada 5SickKids Research Institute, Canada 6Department of Pediatrics, St. Joseph’s Health Centre, Canada Abstract A case of bilateral hilar lymphadenopathy associated with infectious mononucleosis (Epstein-Barr virus infection) in a 9-year-old boy who presented with fever, sore throat, and chest pain is described and published literature on hilar lymphadenopathy in infectious mononucleosis is discussed. This is the first reported case of bilateral hilar lymphadenopathy with Epstein Barr infection in the absence of other pulmonary pathology. It is important for clinicians to be aware of this possibility in children with infectious mononucleosis presenting with chest pain. Keywords: Hilar lymphadenopathy; Epstein-Barr virus; Infectious mononucleosis Abbreviations BHL: Bilateral Hailer Lymphadenopathy; ECG: Electrocardiogram; EBV: Epstein-Barr Virus; ALT: Alanine Transaminase; AST: Aspartate Aminotransferase; EBNA: Anti-Epstein Barr Nuclear Antigen; VCA: Viral Capsid Antibody; IgM: Immunoglobulin M; IgG: Immunoglobulin -

Tooth Pain and Numb Chin As the Initial Presentation of Systemic Malignancy
Tr. J. of Medical Sciences 29 (1999) 719-722 © TÜBİTAK Short Report Sedat ÇETİNER1 Tooth Pain and Numb Chin as the Initial Cansu ALPASLAN1 Nadir GÜNGÖR1 Presentation of Systemic Malignancy Ülker KOÇAK2 1Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, 2Department of Pediatric Haematology, Faculty of Medicine, Gazi University, Ankara-Turkey Received: February 17, 1999 Chin numbness, characterized by numbness of the The initial general features consist of anemia, fever, area innervated by mental nerve is referred to as “numb bleeding tendency, pain, splenomegaly and chin syndrome” (1). This syndrome occurs in association lymphadenopathy accompanied with fatigue. In some rare with the presence of tumors, cysts, inflammatory cases, the initial diagnosis can be made from oral and disorders and trauma. The significance of this syndrome intraoral findings like chin numbness and tooth pain, is its possible relation with neoplastic processes, although they are not pathognomic for this disease. particularly with breast cancer and malignant lymphomas An eleven year-old boy was referred to the (1- 2- 3). Department of Oral and Maxillofacial Surgery, Gazi Acute lymphoblastic leukemia with Burkitt-type cells University, Faculty of Dentistry, in October 1995. His (L3 ALL) was described in 1975. This disease accounts for chief complaint was generalized tooth pain and numbness 1-3 % of all cases of acute lymphoblastic leukemia (ALL) of the lower lip. (4). Figure 1. Intraoral examination of the patient revealing swollen gingiva and plaque accumulation. 719 Tooth Pain and Numb Chin as the Initial Presentation of Systemic Malignancy Figure 2. Periapical radiograph showing the loss of trabecular pattern of bone and loss of lamina dura. -

Primary Syphilis with Oropharyngeal Manifestations Has Traditionally
UC Davis Dermatology Online Journal Title Tonsillar chancre as unusual manifestation of primary syphilis Permalink https://escholarship.org/uc/item/6gn70381 Journal Dermatology Online Journal, 21(4) Authors Lobato-Berezo, Alejandro Imbernon-Moya, Adrian Martinez-Perez, Marcela et al. Publication Date 2015 DOI 10.5070/D3214026282 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 eScholarship.org Powered by the California Digital Library University of California Volume 21 Number 4 April 2015 Letter Tonsillar chancre as unusual manifestation of primary syphilis Alejandro Lobato-Berezo, Adrián Imbernón-Moya, Marcela Martínez-Pérez, Micaela Churruca-Grijelmo, María Elena Vargas-Laguna, Eva Fernández-Cogolludo, Antonio Aguilar-Martínez, Miguel Ángel Gallego- Valdés Dermatology Online Journal 21 (4): 16 1Department of Dermatology, Hospital Universitario Severo Ochoa, Avenida Orellana s/n, Leganés, 28004 Madrid, Spain Correspondence: Alejandro Lobato-Berezo Department of Dermatology Hospital Universitario Severo Ochoa Madrid, Spain [email protected] Abstract Primary syphilis with oropharyngeal manifestations should be kept in mind, though. Lips and tongue ulcers are the most frequently reported lesions and tonsillar ulcers are much more rare. We report the case of a 24-year-old woman with a syphilitic ulcer localized in her left tonsil. Keywords: primary syphilis, tonsil, pharynx, chancre Case synopsis A 24-year-old woman presented to the emergency room complaining of a painless lump on the left side of her tonsil, which started a week before. She was allergic to penicillin and had no relevant medical history. On examination there was a hypertrophic left tonsil with a red-dull aspect and an ulcerated and erosive surface that was deviating the anterior tonsillar pillar (Figure 1).