Composition of Renal Medullary Tissue
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detection of Continuous Erythropoietin Receptor Activator in Blood And
Letters to the Editor 5 A Pugliese-Ciaccio, Catanzaro; Dipartimento di Oncologia, 1.0 Biologia e Genetica Università degli Studi di Genova, Italy. Funding: supported from Associazione Italiana Ricerca sul Cancro 0.8 (AIRC) (to FM and MF) and Fondazione ‘Amelia Scorza’ β2-mneg Onlus, Cosenza, Italy. 0.6 Acknowledgments: we would like to acknowledge Dr. Vincenzo not treated Callea, Prof Luca Baldini, Dr Ugo Consoli and Dr Serena Matis 0.4 for their contribution and useful suggestions.We thank Laura Veroni and Brigida Gulino for precious secretarial assistance. Proportion 0.2 p=0.002 pos β β2-m Key words: 2-microglobulin, CD38, IgVH mutational status, CLL, prognosis. 0.0 Correspondence: Fortunato Morabito, Unità Operativa Complessa di Ematologia, Dipartimento di Medicina Interna, 0 3 6 9 12 15 Azienda Ospedaliera di Cosenza, Viale della Repubblica, years 87100 Cosenza, Italy. Phone: international +39.0984.681329. B Fax: international +39.0984.791751. Univariate analysis E-mail: [email protected] Risk categories HR (95% C.I., p value) Citation: Gentile M, Cutrona G, Neri A, Molica S, Ferrarini M, No factor 1 and Morabito F. Predictive value of B2-microglobulin (B2-m) levels One factor 1,5 (0.7-3.4, p=ns) in chronic lymphocytic leukemia since Binet A stages. Two factor 5.0 (2.5-10.2, p<0.0001) Haematologica 2009; 94:887-888. Three factors 15.4 (7.3-32.5, p<0.0001) doi:10.3324/haematol.2009.005561 C 1.0 References 0.8 1. Rossi D, Zucchetto A, Rossi FM, Capello D, Cerri M, no factor Deambrogi C, et al. -
GFR (Glomerular Filtration Rate) a Key to Understanding How Well Your Kidneys Are Working
GFR (Glomerular Filtration Rate) A Key to Understanding How Well Your Kidneys Are Working www.kidney.org About the Information in this Booklet Did you know that the National Kidney Foundation (NKF) offers guidelines and commentaries that help your healthcare provider make decisions about your medical treatment? The information in this booklet is based on those recommended guidelines. Stages of Kidney Disease There are five stages of kidney disease. They are shown in the table below. Your healthcare provider determines your stage of kidney disease, based on the presence of kidney damage and your glomerular filtration rate (GFR), which is a measure of your kidney function. Your treatment is based on your stage of kidney disease. Speak to your healthcare provider if you have any questions about your stage of kidney disease or your treatment. STAGES OF KIDNEY DISEASE Glomerular Stage Description Filtration Rate (GFR)* Kidney damage (e.g., protein 1 90 or above in the urine) with normal GFR Kidney damage with mild 2 60 to 89 decrease in GFR 3 Moderate decrease in GFR 30 to 59 4 Severe reduction in GFR 15 to 29 5 Kidney failure Less than 15 *Your GFR number tells your healthcare provider how much kidney function you have. As chronic kidney disease progresses, your GFR number decreases. 2 NATIONAL KIDNEY FOUNDATION Why is GFR Important? Most people are aware that their blood pressure and cholesterol numbers are important in knowing their risk for heart and blood vessel disease. Yet few know about glomerular filtration rate (GFR), one of the numbers that tells them about the health of their kidneys. -

Renal Effects of Atrial Natriuretic Peptide Infusion in Young and Adult ~Ats'
003 1-3998/88/2403-0333$02.00/0 PEDIATRIC RESEARCH Vol. 24, No. 3, 1988 Copyright O 1988 International Pediatric Research Foundation, Inc. Printed in U.S.A. Renal Effects of Atrial Natriuretic Peptide Infusion in Young and Adult ~ats' ROBERT L. CHEVALIER, R. ARIEL GOMEZ, ROBERT M. CAREY, MICHAEL J. PEACH, AND JOEL M. LINDEN WITH THE TECHNICAL ASSISTANCE OF CATHERINE E. JONES, NANCY V. RAGSDALE, AND BARBARA THORNHILL Departments of Pediatrics [R.L.C., A.R.G., C.E.J., B. T.], Internal Medicine [R.M.C., J.M. L., N. V.R.], Pharmacology [M.J.P.], and Physiology [J.M.L.], University of Virginia, School of Medicine, Charlottesville, Virginia 22908 ABSTRAm. The immature kidney appears to be less GFR, glomerular filtration rate responsive to atrial natriuretic peptide (ANP) than the MAP, mean arterial pressure mature kidney. It has been proposed that this difference UeC~pV,urinary cGMP excretion accounts for the limited ability of the young animal to UN,V, urinary sodium excretion excrete a sodium load. To delineate the effects of age on the renal response to exogenous ANP, Sprague-Dawley rats were anesthetized for study at 31-32 days of age, 35- 41 days of age, and adulthod. Synthetic rat A* was infused intravenously for 20 min at increasing doses rang- By comparison to the adult kidney, the immature kidney ing from 0.1 to 0.8 pg/kg/min, and mean arterial pressure, responds to volume expansion with a more limited diuresis and glomerular filtration rate, plasma ANP concentration, natriuresis (I). A number of factors have been implicated to urine flow rate, and urine sodium excretion were measured explain this phenomenon in the neonatal kidney, including a at each dose. -

1. Urine Diversion
1. Urine diversion – hygienic risks and microbial guidelines for reuse © Caroline Schönning Department of Parasitology, Mycology and Environmental Microbiology Swedish Institute for Infectious Disease Control (SMI) SE-171 82 Solna Sweden [email protected] This chapter is based on the doctoral thesis published by the author in February 2001: Höglund, C. (2001). Evaluation of microbial health risks associated with the reuse of source separated human urine. PhD thesis, Department of Biotechnology, Royal Institute of Technology, Stockholm, Sweden. ISBN 91-7283-039-5. The full thesis (87 pages, without published papers) can be downloaded from: http://www.lib.kth.se/Sammanfattningar/hoglund010223.pdf Dr Håkan Jönsson, Swedish University for Agricultural Sciences is acknowledged for compiling Section 3, and Dr Jan-Olof Drangert, Linköping University is acknowledged for compiling Section 9. TABLE OF CONTENTS TABLE OF CONTENTS 1 1. INTRODUCTION 2 1.1 History 2 1.2 Nutrient content and volume of domestic wastewater 3 2. URINE DIVERSION 3 2.1 Urine diversion in Sweden 4 2.2 Source-separation of urine in other parts of the world 6 2.3 Ecological Sanitation 6 3. URINE AS A FERTILISER IN AGRICULTURE 7 3.1 Characteristics of diverted human urine 7 3.2 Collection and storage of the urine – developing countries 7 3.3 Urine as a fertiliser 8 3.4 Crops to fertilise 9 3.5 Dosage 9 3.6 Fertilising experiments 10 3.7 Acceptance 11 4. PATHOGENIC MICROORGANISMS IN URINE 11 5. FAECAL CONTAMINATION 13 5.1 Analysis of indicator bacteria to determine faecal contamination 14 5.2 Analysis of faecal sterols to determine faecal contamination 15 5.3 Discussion 16 6. -
Urinalysis and Kidney Disease: What You Need to Know
URINALYSIS AND KIDNEY DISEASE What You Need To Know www.kidney.org About the Information in this Booklet Did you know that the National Kidney Foundation (NKF) offers guidelines and commentaries that help your healthcare provider make decisions about your medical treatment? The information in this booklet is based on those recommended guidelines. Stages of Kidney Disease There are five stages of kidney disease. They are shown in the table below. Your healthcare provider determines your stage of kidney disease based on the presence of kidney damage and your glomerular filtration rate (GFR), which is a measure of your kidney function. Your treatment is based on your stage of kidney disease. Speak to your healthcare provider if you have any questions about your stage of kidney disease or your treatment. STAGES OF KIDNEY DISEASE Glomerular Stage Description Filtration Rate (GFR)* Kidney damage (e.g., protein 1 90 or above in the urine) with normal GFR Kidney damage with mild 2 60 to 89 decrease in GFR 3 Moderate decrease in GFR 30 to 59 4 Severe reduction in GFR 15 to 29 5 Kidney failure Less than 15 *Your GFR number tells your healthcare provider how much kidney function you have. As chronic kidney disease progresses, your GFR number decreases. What is a urinalysis (also called a “urine test”)? A urinalysis is a simple test that looks at a small sample of your urine. It can help find conditions that may need treatment, including infections or kidney problems. It can also help find serious diseases in the early stages, like chronic kidney disease, diabetes, or liver disease. -

The Urinary System Dr
The urinary System Dr. Ali Ebneshahidi Functions of the Urinary System • Excretion – removal of waste material from the blood plasma and the disposal of this waste in the urine. • Elimination – removal of waste from other organ systems - from digestive system – undigested food, water, salt, ions, and drugs. + - from respiratory system – CO2,H , water, toxins. - from skin – water, NaCl, nitrogenous wastes (urea , uric acid, ammonia, creatinine). • Water balance -- kidney tubules regulate water reabsorption and urine concentration. • regulation of PH, volume, and composition of body fluids. • production of Erythropoietin for hematopoieseis, and renin for blood pressure regulation. Anatomy of the Urinary System Gross anatomy: • kidneys – a pair of bean – shaped organs located retroperitoneally, responsible for blood filtering and urine formation. • Renal capsule – a layer of fibrous connective tissue covering the kidneys. • Renal cortex – outer region of the kidneys where most nephrons is located. • Renal medulla – inner region of the kidneys where some nephrons is located, also where urine is collected to be excreted outward. • Renal calyx – duct – like sections of renal medulla for collecting urine from nephrons and direct urine into renal pelvis. • Renal pyramid – connective tissues in the renal medulla binding various structures together. • Renal pelvis – central urine collecting area of renal medulla. • Hilum (or hilus) – concave notch of kidneys where renal artery, renal vein, urethra, nerves, and lymphatic vessels converge. • Ureter – a tubule that transport urine (mainly by peristalsis) from the kidney to the urinary bladder. • Urinary bladder – a spherical storage organ that contains up to 400 ml of urine. • Urethra – a tubule that excretes urine out of the urinary bladder to the outside, through the urethral orifice. -

Urine Filtration Part 3: Session Activities
Urine Filtration The urine filtration test is used to identify and quantify S. haematobium eggs in urine; this tool is commonly used in SCH control programmes as it is one of the few methods available in the field. The tool is a fast and easy technique which uses 10mL of urine, the sample is passed through a nucleopore filter which can then be viewed under a microscope at 40x with iodine to determine the intensity of the S. haematobium infection. It is important to note that the reading should be recorded as the number of eggs per the volume used; this will usually be number of eggs/10mL. Part 3: Session Activities Activity 1: Sharing experiences Participants will form into groups of 4-6 individuals and will discuss their experiences with the current laboratory and diagnostic tools that they have used in the past and are familiar with. Groups should highlight the following: The tools they have used The challenges associated with each tool The gaps that they feel exist in NTD laboratory and diagnostic tools After 10 minutes each group will discuss the key points that they have highlighted with the facilitators and other participants. The aim of this activity is for participants to become familiar with the challenges that other individuals in the NTD community have faced in regard to laboratory and diagnostic tools. Activity 2: Case study In DRC, in the provinces of Equateur and Bas Congo, most of the districts are co-endemic to LF, Loa loa and Onchocerciasis. While completing mapping for LF, Loa loa and Onchocerciasis, could you describe the diagnostic methods that should be used to confirm endemicity to LF as we know that MDA with Ivermectin could not be implemented in districts where LF and Loa loa are endemic? Which methods will you carry out to map LF? Which diagnostic methods will you use to map Loa loa? Which additional laboratory and diagnostic methods will you use to confirm endemicity of LF and Loa loa in most of the districts? For each diagnostic and lab methods please explain your choice. -

Water Deprivation Test and Desmopressin Test in Adults
BLOOD SCIENCES DEPARTMENT OF CLINICAL BIOCHEMISTRY Title of Document: Water Deprivation Test in Adults Q Pulse Reference No: BS/CB/DCB/EN/16 Version NO: 5 Authoriser: Sadie Redding Page 1 of 4 WATER DEPRIVATION TEST Introduction Diabetes insipidus (DI) involves deficient production or lack of effective action of antidiuretic hormone (ADH or arginine vasopressin). ADH stimulates the kidney to conserve fluid. Deficient production of ADH or lack of effective action of ADH causes a high urine output, thirst, dehydration, and low blood pressure in advanced cases. Disease of the hypothalamus/pituitary gland leading to a deficiency in ADH production is called cranial or central DI. Disease of the kidney leading to lack of response of the kidney to fluid conserving action of ADH is called nephrogenic DI. The principle of the water deprivation test is to assess the ability of the patient to concentrate urine when fluids are withheld. Water deprivation should normally cause increased secretion of ADH, resulting in the production of small volumes of concentrated urine. Initial tests Polyuria should be confirmed by 24hour urine volume. Rule out diabetes, UTI, hypercalcaemia, hypokalaemia, renal failure and thyrotoxicosis. Morning urine osmolality >600mOsm/kg rules out DI and therefore a water deprivation test is not necessary. Preparation Please contact the Duty Biochemist at least 24 hours before the test (ext.48437) Normal dinner and fluids are allowed the night before the test. No alcohol or caffeine. 7am light breakfast but NO fluids, tea, coffee or smoking. Supervision is required throughout to assess compliance and safety. Contraindications Hypovolaemia or hypernatraemia Procedure Print out a worksheet and record results in the table (appendix 1). -

Arginine Vasopressin, Plasma Vasopressin, Arginine • • •
Arginine Vasopressin, Plasma 252X Clinical Use Clinical Background • Diagnose central diabetes insipidus Arginine vasopressin (AVP), or (DI) antidiuretic hormone (ADH), is a nonapeptide produced by the hypo- • Differential diagnosis of central DI vs Test Alphabetical nephrogenic DI thalamus and released from the posterior pituitary in response to • Diagnose syndrome of inappropriate extracellular fluid hyperosmolarity and Section ADH secretion (SIADH) hypovolemia. AVP promotes concen- tration of the urine by increasing water Reference Range reabsorption in the kidney tubules. 1.0-13.3 pg/mL Inadequate AVP action causes Note: 2.5 pg = 1 μU diabetes insipidus (DI), a syndrome characterized by nonglycosuric Interpretive Information polyuria, polydipsia, and dehydration. • SIADH Central DI refers to insufficient AVP release due to diseases of the hypo- • Ectopic ADH syndrome thalamus, pituitary stalk, and pituitary • Nephrogenic DI gland. Nephrogenic DI is the result of • Phenothiazine, carbamazepine impaired renal responsiveness to AVP and may be congenital or due to renal • Central DI disease, hypokalemia, hypercalcemia, systemic disorders (eg, multiple myeloma and amyloidosis), or drugs (eg, lithium or demeclocycline and ethanol). DI diagnosis is based on the presence of hyperosmolar serum with in- appropriately dilute urine. Central and nephrogenic DI can be differentiated by measuring the plasma AVP level and interpreting it in light of the simul- taneous plasma osmolality. The syndrome of inappropriate secretion of antidiuretic hormone (SIADH) is manifest by hyponatremia and inappropriately concentrated urine. The diagnosis is confirmed by plasma or urine AVP levels in- appropriate for serum osmolality. Method • Extraction, radioimmunoassay (RIA) • Analytical sensitivity: 1.0 pg/mL Specimen Requirements 4 mL frozen EDTA plasma 1.1 mL minimum Draw blood in prechilled tube; centrifuge immediately in refrigerated centrifuge. -

General Urine Specimen Collection Procedure
Blanchard Valley Health System Laboratory Servies Blanchard Valley Hospital Bluffton Community Hospital 1900 South Main Street, Findlay, OH 45840 139 Garau Street, Bluffton, OH. 45817 General Urine Specimen Collection Procedure (LTR27570) Last Approved By: Hughes, Douglas (2/3/2016 5:45:03 AM) Revision: 2.01 Last Approved Time: 2/3/2016 5:45:03 AM Attention: Printed copies MAY not be the most current information. Please consult the Lab QMS for the current version. Urine Specimen Collection Procedure POSTED-LIS PRINCIPLE: Urine is one type of specimen that can be easily collected from a patient. Urinalysis testing can give the doctor valuable information about many body systems especially kidney function. The physician uses the information from urine testing to diagnose and treat many disease states. Types of urine specimens: 1. Random sample: Sample which is collected anytime during the day. Usually used only for routine screening because the composition of urine changes throughout the day. 2. First voided specimen: Sample also referred to as a first morning specimen. This sample is collected the first time the patient urinates in the morning. A first voided specimen is the most concentrated and is the preferred specimen for pregnancy testing, bacterial cultures and microscopic examinations. 3. Timed specimens: These specimens are used when the physician requires urine samples to be taken at specific intervals during the day. Twenty-four (24) hour urine specimens are required for creatinine clearance tests and many other hormone studies. 4. Clean-catch midstream specimen: This sample type is collected if the urine is going to be cultured and examined for bacterial growth or used for cytology. -
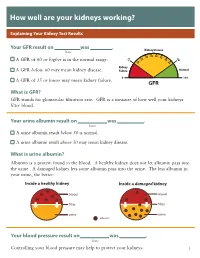
How Well Are Your Kidneys Working?
How well are your kidneys working? Explaining Your Kidney Test Results Kidney Your GFR result on was . Kidney Disease Date 35 40 0 45 3 6 5 5 50 0 A GFR of 60 or higher is in the normal range. 1 2 5 0 5 2 Kidney A GFR below 60 may mean kidney disease. Failure Normal 0 120 A GFR of 15 or lower may mean kidney failure. GFR What is GFR? GFR stands for glomerular filtration rate. GFR is a measure of how well your kidneys filter blood. Your urine albumin result on was . Date A urine albumin result below 30 is normal. A urine albumin result above 30 may mean kidney disease. What is urine albumin? Albumin is a protein found in the blood. A healthy kidney does not let albumin pass into the urine. A damaged kidney lets some albumin pass into the urine. The less albumin in your urine, the better. Inside a healthy kidney Inside a damaged kidney blood blood filter filter urine urine albumin Your blood pressure result on was . Date Controlling your blood pressure may help to protect your kidneys. 1 What your kidneys do You have two kidneys. Their main job is to filter wastes and extra water out of your blood to make urine. kidneys How your kidneys are checked Two tests are used to check for kidney disease. • A blood test checks your GFR, which tells how well your kidneys are filtering. • A urine test checks for albumin in your urine, a sign of kidney damage. Why your kidneys are being checked You need to have your kidneys checked because you can’t feel kidney disease. -

Renal Physiology and Pathophysiology of the Kidney
Renal Physiology and pathophysiology of the kidney Alain Prigent Université Paris-Sud 11 IAEA Regional Training Course on Radionuclides in Nephrourology Mikulov, 10–11 May 2010 The glomerular filtration rate (GFR) may change with - The adult age ? - The renal plasma (blood) flow ? + - The Na /water reabsorption in the nephron ? - The diet variations ? - The delay after a kidney donation ? IAEA Regional Training Course on Radionuclides in Nephrourology Mikulov, 10–11 May 2010 GFR can measure with the following methods - The Cockcroft-Gault formula ? - The urinary creatinine clearance ? - The Counahan-Baratt method in children? - The Modification on Diet in Renal Disease (MDRD) formula in adults ? - The MAG 3 plasma sample clearance ? IAEA Regional Training Course on Radionuclides in Nephrourology Mikulov, 10–11 May 2010 About the determinants of the renogram curve (supposed to be perfectly « BKG » corrected) 99m -The uptake (initial ascendant segment) of Tc DTPA depends on GFR 99m -The uptake (initial ascendant segment) of Tc MAG 3 depends almost only on renal plasma flow 123 -The uptake (initial ascendant segment) of I hippuran depends both on renal plasma flow and GFR -The height of renogram maximum (normalized to the injected activity) reflects on the total nephron number -The « plateau » pattern of the late segment of the renogram does mean obstruction ? IAEA Regional Training Course on Radionuclides in Nephrourology Mikulov, 10–11 May 2010 Overview of the kidney functions Regulation of the volume and composition of the body fluids