Saraji East Mining Lease Project Environmental Impact Statement
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Australasian Bat Society Newsletter, Number 31, Nov 2008
The Australasian Bat Society Newsletter, Number 31, Nov 2008 The Australasian Bat Society Newsletter Number 39 November 2012 ABS Website: http://abs.ausbats.org.au ABS Discussion list - email: [email protected] ISSN 1448-5877 © Copyright The Australasian Bat Society, Inc. (2012) The Australasian Bat Society Newsletter, Number 31, Nov 2008 The Australasian Bat Society Newsletter, Number 39, November 2012 – Instructions for Contributors – The Australasian Bat Society Newsletter will accept contributions under one of the following two sections: Research Papers, and all other articles or notes. There are two deadlines each year: 10th March for the April issue, and 10th October for the November issue. The Editor reserves the right to hold over contributions for subsequent issues of the Newsletter, and meeting the deadline is not a guarantee of immediate publication. Opinions expressed in contributions to the Newsletter are the responsibility of the author, and do not necessarily reflect the views of the Australasian Bat Society, its Executive or members. For consistency, the following guidelines should be followed: Emailed electronic copy of manuscripts or articles, sent as an attachment, is the preferred method of submission. Faxed and hard copy manuscripts will be accepted but reluctantly! Please send all submissions to the Newsletter Editor at the email or postal address below. Electronic copy should be in 11 point Arial font, left and right justified with 16 mm left and right margins. Please use Microsoft Word; any version is acceptable. Manuscripts should be submitted in clear, concise English and free from typographical and spelling errors. Please leave two spaces after each sentence. -

Brigalow Belt Bioregion – a Biodiversity Jewel
Brigalow Belt bioregion – a biodiversity jewel Brigalow habitat © Craig Eddie What is brigalow? including eucalypt and cypress pine forests and The term ‘brigalow’ is used simultaneously to refer to; woodlands, grasslands and other Acacia dominated the tree Acacia harpophylla; an ecological community ecosystems. dominated by this tree and often found in conjunction with other species such as belah, wilga and false Along the eastern boundary of the Brigalow Belt are sandalwood; and a broader region where this species scattered patches of semi-evergreen vine thickets with and ecological community are present. bright green canopy species that are highly visible among the more silvery brigalow communities. These The Brigalow Belt bioregion patches are a dry adapted form of rainforest, relics of a much wetter past. The Brigalow Belt bioregion is a large and complex area covering 36,400 000ha. The region is thus recognised What are the issues? by the Australian Government as a biodiversity hotspot. Nature conservation in the region has received increasing attention because of the rapid and extensive This hotspot contains some of the most threatened loss of habitat that has occurred. Since World War wildlife in the world, including populations of the II the Brigalow Belt bioregion has become a major endangered bridled nail-tail wallaby and the only agricultural and pastoral area. Broad-scale clearing for remaining wild population of the endangered northern agriculture and unsustainable grazing has fragmented hairy-nosed wombat. The area contains important the original vegetation in the past, particularly on habitat for rare and threatened species including the, lowland areas. glossy black-cockatoo, bulloak jewel butterfl y, brigalow scaly-foot, red goshawk, little pied bat, golden-tailed geckos and threatened community of semi evergreen Biodiversity hotspots are areas that support vine thickets. -

Chiropterology Division BC Arizona Trial Event 1 1. DESCRIPTION: Participants Will Be Assessed on Their Knowledge of Bats, With
Chiropterology Division BC Arizona Trial Event 1. DESCRIPTION: Participants will be assessed on their knowledge of bats, with an emphasis on North American Bats, South American Microbats, and African MegaBats. A TEAM OF UP TO: 2 APPROXIMATE TIME: 50 minutes 2. EVENT PARAMETERS: a. Each team may bring one 2” or smaller three-ring binder, as measured by the interior diameter of the rings, containing information in any form and from any source. Sheet protectors, lamination, tabs and labels are permitted in the binder. b. If the event features a rotation through a series of stations where the participants interact with samples, specimens or displays; no material may be removed from the binder throughout the event. c. In addition to the binder, each team may bring one unmodified and unannotated copy of either the National Bat List or an Official State Bat list which does not have to be secured in the binder. 3. THE COMPETITION: a. The competition may be run as timed stations and/or as timed slides/PowerPoint presentation. b. Specimens/Pictures will be lettered or numbered at each station. The event may include preserved specimens, skeletal material, and slides or pictures of specimens. c. Each team will be given an answer sheet on which they will record answers to each question. d. No more than 50% of the competition will require giving common or scientific names. e. Participants should be able to do a basic identification to the level indicated on the Official List. States may have a modified or regional list. See your state website. -

Wildlife Matters Wildlife Conservancy
australian wildlife matters wildlife conservancy Spring 2009 Pungalina reveals one of Australia’s rarest mammals Carpentarian Pseudantechinus 2 australian saving australia’s threatened wildlife wildlife Pictograph conservancy Welcome to the Spring 2009 edition of Wildlife Matters. As this edition goes to print, we are in the process of fi nalising the acquisition of Bowra (see pages 4-5), a 14,000 the awc mission hectare property located in the heart of the Mulga Lands in Queensland. Bowra will The mission of Australian Wildlife Conservancy be our 21st sanctuary, bringing the AWC network to more than 2.56 million hectares (AWC) is the effective conservation of all (6.3 million acres). Australian animal species and the habitats in While the overall scale of the portfolio is impressive, it is not the number of properties or which they live. To achieve this mission, our hectares that really count. A more accurate measure of the value of the portfolio is the actions are focused on: number of species and ecosystems that occur within the AWC estate. In this respect, • Establishing a network of sanctuaries the statistics are even more impressive – for example, around 80% of all Australian which protect threatened wildlife and terrestrial bird species and over 60% of all terrestrial mammal species occur on one or ecosystems: AWC now manages 20 more of our sanctuaries. sanctuaries covering over 2.56 million The fact that our portfolio captures such a high percentage of Australia’s wildlife species hectares (6.3 million acres). refl ects a deliberate, science-based strategy to ensure that AWC invests in properties • Implementing practical, on-ground of the highest environmental value. -

Terrestrial Fauna Assessment
Cameby Downs Continued Operations Project EnvironmentalEnvironmental Values Assessment Assessment APPENDIX E Terrestrial Fauna Assessment Cameby Downs Continued Operation Project Terrestrial Fauna Assessment May 2018 Syntech Resources Pty Ltd ecology / vegetation / wildlife / aquatic ecology / GIS Executive summary The Cameby Downs Mine is owned and operated by Syntech Resources Pty Ltd (Syntech) and is managed by Yancoal Australia Ltd (Yancoal). Syntech are considering expanding their operation area as part of the Cameby Downs Continued Operations Project (the Project) and an environmental values statement is being prepared to accompany a major Environmental Authority Amendment application. Syntech commissioned Ecosure Pty Ltd (Ecosure) to undertake terrestrial fauna field surveys and ecological assessments to address the minimum requirements in the Queensland Department of Environment and Heritage (DEHP) Information Request for an Amendment Application for an Environmental Authority. To supplement previous fauna surveys undertaken over the last decade, Ecosure undertook preliminary surveys and targeted surveys in July 2016. More comprehensive surveys followed in October 2016. Overall, six detailed trapping sites, 50 observational surveys and 56 targeted surveys were undertaken across the study area to determine the likelihood of occurrence of species listed as conservation significant species under State legislation and/ or threatened under Commonwealth legislation. A total of five conservation significant species have been recorded during field surveys (including previous surveys) conducted in the study area. These were: • koala (Phascolarctos cinereus) • glossy black-cockatoo (Calyptorhynchus lathami lathami) • grey snake (Hemiaspis damelii) • short-beaked echidna (Tachyglossus aculeatus) • yakka skink (Egernia rugosa) (during previous surveys only). A total of 192 species were recorded during the field surveys, including 13 amphibians, 101 birds, 25 mammals and 26 reptiles. -

Structure±Function Properties of Venom Components from Australian Elapids
PERGAMON Toxicon 37 (1999) 11±32 Review Structure±function properties of venom components from Australian elapids Bryan Grieg Fry * Peptide Laboratory, Centre for Drug Design and Development, University of Queensland, St. Lucia, Qld, 4072, Australia Received 9 December 1997; accepted 4 March 1998 Abstract A comprehensive review of venom components isolated thus far from Australian elapids. Illustrated is that a tremendous structural homology exists among the components but this homology is not representative of the functional diversity. Further, the review illuminates the overlooked species and areas of research. # 1998 Elsevier Science Ltd. All rights reserved. 1. Introduction Australian elapids are well known to be the most toxic in the world, with all of the top ten and nineteen of the top 25 elapids with known LD50s residing exclusively on this continent (Broad et al., 1979). Thus far, three main types of venom components have been characterised from Australian elapids: prothrombin activating enzymes; lipases with a myriad of potent activities; and powerful peptidic neurotoxins. Many species have the prothrombin activating enzymes in their venoms, the vast majority contain phospholipase A2s and all Australian elapid venoms are suspected to contain peptidic neurotoxins. In addition to the profound neurological eects such as disorientation, ¯accid paralysis and respiratory failure, characteristic of bites by many species of Australian elapids is hemorrhaging and incoagulable blood. As a result, these elapids can be divided into two main classes: species with procoagulant venom (Table 1) and species with non-procoagulant venoms (Table 2) (Tan and * Author to whom correspondence should be addressed. 0041-0101/98/$ - see front matter # 1998 Elsevier Science Ltd. -

Code of Practice Captive Reptile and Amphibian Husbandry Nature Conservation Act 1992
Code of Practice Captive Reptile and Amphibian Husbandry Nature Conservation Act 1992 ♥ The State of Queensland, Department of Environment and Science, 2020 Copyright protects this publication. Except for purposes permitted by the Copyright Act, reproduction by whatever means is prohibited without prior written permission of the Department of Environment and Science. Requests for permission should be addressed to Department of Environment and Science, GPO Box 2454 Brisbane QLD 4001. Author: Department of Environment and Science Email: [email protected] Approved in accordance with section 174A of the Nature Conservation Act 1992. Acknowledgments: The Department of Environment and Science (DES) has prepared this code in consultation with the Department of Agriculture, Fisheries and Forestry and recreational reptile and amphibian user groups in Queensland. Human Rights compatibility The Department of Environment and Science is committed to respecting, protecting and promoting human rights. Under the Human Rights Act 2019, the department has an obligation to act and make decisions in a way that is compatible with human rights and, when making a decision, to give proper consideration to human rights. When acting or making a decision under this code of practice, officers must comply with that obligation (refer to Comply with Human Rights Act). References referred to in this code- Bustard, H.R. (1970) Australian lizards. Collins, Sydney. Cann, J. (1978) Turtles of Australia. Angus and Robertson, Australia. Cogger, H.G. (2018) Reptiles and amphibians of Australia. Revised 7th Edition, CSIRO Publishing. Plough, F. (1991) Recommendations for the care of amphibians and reptiles in academic institutions. National Academy Press: Vol.33, No.4. -

A Preliminary Assessment of Faunal Values Within and Adjacent EPC 1029, Styx Basin, Central-East Queensland
A preliminary assessment of faunal values within and adjacent EPC 1029, Styx Basin, central-east Queensland ) Prepared for Yeats Consulting Engineers by Ed Meyer, Ecological Consultant,S Luscombe Street, Runcorn QLD 4113 ([email protected]) Conditions of use This report may only be used for the purposes for which it was commissioned. The use of this report, or part thereof, for any other reason or purpose is prohibited without the written consent of the author. Front cover: Fauna recorded from EPC 1029 during March 2011 surveys. Clockwise from upper left: ornamental snake (Denisonia maculata); squatter pigeon (southern race) (Geophaps scripta scripta); metallic snake-eyed skink (Cryptoblepharus metal/icus); and eastern sedgefrog (Litoria tal/ax). ©Edward Meyer 2011 5 Luscombe Street, Runcorn QLD 4113 E-mail:[email protected] Version 2 _ 3 August 2011 2 Table of contents 1. Summary 4 2. Background 6 Description of study area 6 Nomenclature 6 Abbreviations and acronyms 7 3. Methodology 9 General approach 9 ) Desktop assessment 9 Likelihood of occurrence assessments 10 Field surveys 11 Survey conditions 15 Survey limitations 15 4. Results 17 Desktop assessment findings 17 Likelihood of occurrence assessments 17 Field survey results -fauna 20 Field survey results - fauna habitat 22 Habitat for conservation significant species 28 ) 5. Summary and conclusions 37 6. References 38 Appendix A: Fauna previously recorded from Desktop Assessment Study Area 41 Appendix B: likelihood of occurrence assessments for conservation significant fauna 57 Appendix C: March 2011 survey results 73 Appendix D: Habitat photos 85 Appendix E: Habitat assessment proforma 100 3 1. Summary The faunal values of land within and adjacent Exploration Permit for Coal (EPe) 1029 were investigated by way of desktop review of existing information as well as field surveys carried out in late March 201l. -
Eton Range Realignment Project ATTACHMENT 2 to EPBC Ref: 2015/7552 Preliminary Documentation Residual Impact Assessment and Offset Proposal - 37
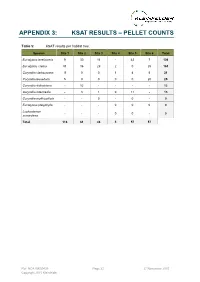
APPENDIX 3: KSAT RESULTS – PELLET COUNTS Table 5: KSAT results per habitat tree. Species Site 1 Site 2 Site 3 Site 4 Site 5 Site 6 Total Eucalyptus tereticornis 9 30 16 - 42 7 104 Eucalyptus crebra 91 16 29 2 0 25 163 Corymbia clarksoniana 11 0 0 1 4 5 21 Corymbia tessellaris 5 0 0 0 0 20 25 Corymbia dallachiana - 12 - - - - 12 Corymbia intermedia - 3 1 0 11 - 15 Corymbia erythrophloia - - 0 - 0 - 0 Eucalyptus platyphylla - - - 0 0 0 0 Lophostemon - - - 0 0 - 0 suaveolens Total 116 61 46 3 57 57 Ref: NCA15R30439 Page 22 27 November 2015 Copyright 2015 Kleinfelder APPENDIX 4: SITE PHOTOS The following images were taken from the centre of each BioCondition quadrat and represent a north east south west aspect, top left to bottom right. Ref: NCA15R30439 Page 23 27 November 2015 Copyright 2015 Kleinfelder Plate 3: BioCondition quadrat 1 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 24 27 November 2015 Copyright 2015 Kleinfelder Plate 4: BioCondition quadrat 2 (RE11.3.4/11.12.3) Ref: NCA15R30439 Page 25 27 November 2015 Copyright 2015 Kleinfelder Plate 5: BioCondition quadrat 3 (RE11.12.3) Ref: NCA15R30439 Page 26 27 November 2015 Copyright 2015 Kleinfelder Plate 6: BioCondition quadrat 4 (RE11.3.9) Ref: NCA15R30439 Page 27 27 November 2015 Copyright 2015 Kleinfelder Plate 7: BioCondition quadrat 5 (RE11.3.25) Ref: NCA15R30439 Page 28 27 November 2015 Copyright 2015 Kleinfelder Plate 8: BioCondition quadrat 6 (RE11.12.3/11.3.4/11.3.9) Ref: NCA15R30439 Page 29 27 November 2015 Copyright 2015 Kleinfelder Appendix E: Desktop Assessment for Potential -

Article (Published Version)
Article Identifying the snake: First scoping review on practices of communities and healthcare providers confronted with snakebite across the world BOLON, Isabelle, et al. Abstract Background Snakebite envenoming is a major global health problem that kills or disables half a million people in the world’s poorest countries. Biting snake identification is key to understanding snakebite eco-epidemiology and optimizing its clinical management. The role of snakebite victims and healthcare providers in biting snake identification has not been studied globally. Objective This scoping review aims to identify and characterize the practices in biting snake identification across the globe. Methods Epidemiological studies of snakebite in humans that provide information on biting snake identification were systematically searched in Web of Science and Pubmed from inception to 2nd February 2019. This search was further extended by snowball search, hand searching literature reviews, and using Google Scholar. Two independent reviewers screened publications and charted the data. Results We analysed 150 publications reporting 33,827 snakebite cases across 35 countries. On average 70% of victims/bystanders spotted the snake responsible for the bite and 38% captured/killed it and brought it to the healthcare facility. [...] Reference BOLON, Isabelle, et al. Identifying the snake: First scoping review on practices of communities and healthcare providers confronted with snakebite across the world. PLOS ONE, 2020, vol. 15, no. 3, p. e0229989 PMID : 32134964 DOI : 10.1371/journal.pone.0229989 Available at: http://archive-ouverte.unige.ch/unige:132992 Disclaimer: layout of this document may differ from the published version. 1 / 1 PLOS ONE RESEARCH ARTICLE Identifying the snake: First scoping review on practices of communities and healthcare providers confronted with snakebite across the world 1 1 1 1,2 Isabelle BolonID *, Andrew M. -

Timothy Wilson Roads and Maritime Services Level Ground 1 Simmons Street, Wagga Wagga NSW 2650
P.O. Box 1088 Newport, Victoria 3015 Phone: 03 9391 4749 Mobile: 0414 689 853 Email: [email protected] ABN: 811 5163 3797 Timothy Wilson Roads and Maritime Services Level Ground 1 Simmons Street, Wagga Wagga NSW 2650 5/04/2015 RE: Echuca Bat Call Peer Review; Contract No. 15.2571.0265 Dear Timothy, As requested, I have undertaken a review of the bat sonograms that were attributed to Nyctophilus corbeni following the survey undertaken at the site of the proposed new Echuca - Moama Bridge. I am of the belief that the call parameter employed by Dr Greg Richards to identify Nyctophilus corbeni sonograms (minimum frequency of 35kHz) is not a reliable identification parameter. The reference calls of two species, being N. geoffroyi and N. gouldi (captured in the Echuca - Moama region) also display calls at a minimum frequency of 35kHz or lower. Six subject matter experts were consulted on whether individual Nyctophilus species could be identified from a specific call parameter. All six experts were of the opinion that with the current technology available for recording bat calls, it is not possible to identify Nyctophilus from their call alone. A review of the Victorian Biodiversity Atlas, BioNet Wildlife Atlas of NSW and Atlas of Living Australia provided historical records of N. corbeni. With the exception of the record submitted by Dr Richards to the BioNet Atlas of NSW, the next nearest individual records to the site are near Deniliquin in NSW (70km north of Echuca) and Mitiamo in Victoria (50km west of Echuca). A number of the subject matter experts with whom I consulted during the preparation of this review have extensive trapping experience in the Echuca – Moama region, and are yet to record N. -

Carmichael Conservation and Research Program
Carmichael Conservation and Research Program 3 April 2019 Version 6 1 Carmichael Conservation and Research Program Contents 1 Introduction ............................................................................................................................... 5 2 Carmichael Conservation and Research Program ....................................................................... 5 2.1 Objective ........................................................................................................................... 5 2.2 Descriptions of Matters of National Environmental Significance ........................................ 7 2.2.1 Black-throated Finch (southern) .................................................................................. 7 2.2.2 Brigalow Threatened Ecological Community .............................................................. 7 2.2.3 Ornamental snake ....................................................................................................... 8 2.2.4 Squatter pigeon (southern) .......................................................................................... 8 2.2.5 Waxy cabbage palm ................................................................................................... 9 2.2.6 Yakka skink ............................................................................................................... 9 2.3 Species recovery information - threats .............................................................................. 10 2.4 Existing research gap analysis .........................................................................................