Pathology of Paediatric Bone Tumours
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Musculoskeletal Tumor Information
Tumor Information Bone Tumors Soft Tissue Tumors Bone Tumors Bone tumors are a rare cause of musculoskeletal pain but should always be considered in the patient with otherwise unexplained pain. Most bone tumors present with pain and/or a mass. Care must be taken to ensure the correct diagnosis is made, and early consultation with an orthopaedic oncologist is advised to avoid potential complications. In general, these tumors are best treated at a referral practice that specializes in bone tumors. Benign Presenting symptoms Benign bone tumors can have a wide variety of presenting symptoms; in general, benign bone tumors present with pain. Tumors can occur in any bone, and can occur in all age groups. In general, these tumors are a rare cause of musculoskeletal pain, but should be considered when the diagnosis is in question. Often, benign tumors are found incidentally when patients are imaged for other reasons (i.e., a football player hurts his knee and gets an X- ray to rule out fracture; a suspicious tumor is seen). These are usually benign tumors, but need to be carefully evaluated by an orthopaedic tumor specialist. Diagnostic Imaging Imaging is necessary to diagnose a bone tumor. Often, multiple tests are ordered, but must be evaluated carefully by an orthopaedic tumor specialist to make sure that the most accurate diagnosis is rendered. X-Ray Usually done in the office, this is the most basic imaging test. Plain X- rays can provide essential diagnostic information, and must be of high quality. It is not uncommon to have to repeat these in order to make sure a high quality digital image is obtained. -

Bone & Soft Tissue
14A ANNUAL MEETING ABSTRACTS and testicular atrophy with aspermatogenia (negative OCT3/OCT4 stain). There was 47 Comparison of Autopsy Findings of 2009 Pandemic Influenza evidence of acute multifocal bronchopneumonia and congestive heart failure. He carried A (H1N1) with Seasonal Influenza in Four Pediatric Patients two heterozygous mutations in ALMS1: 11316_11319delAGAG; R3772fs in exon 16 B Xu, JJ Woytash, D Vertes. State University of New York at Buffalo, Buffalo, NY; Erie and 8164C>T ter; R2722X in exon 10. County Medical Examiner’s Office, Buffalo, NY. Conclusions: This report describes previously undefined cardiac abnormalities in this Background: The swine-origin influenza A (H1N1) virus that emerged in humans rare multisystem disorder. Myofibrillar disarray is probably directly linked to ALMS1 in early 2009 has reached pandemic proportions and cause over 120 pediatric deaths mutation, while fibrosis in multiple organs may be a secondary phenomenon to gene nationwide. Studies in animal models have shown that the 2009 H1N1 influenza virus alteration. Whether and how intracellular trafficking or related signals lead to cardiac is more pathogenic than seasonal A virus, with more extensive virus replication and dysfunction is a subject for further research. shedding occurring the respiratory tract. Design: We report four cases of influenza A-associated deaths (two pandemic and two 45 Sudden Cardiac Death in Young Adults: An Audit of Coronial seasonal) in persons less than fifteen years of age who had no underlying health issues. Autopsy Findings Autopsy finding on isolation of virus from various tissue specimen, cocurrent bacterial A Treacy, A Roy, R Margey, JC O’Keane, J Galvin, A Fabre. -

Musculoskeletal
MUSCULOSKELETAL Dr. Dean Bruce University of Alberta DISCLOSURES… Member of the Royal College Examination Committee in Diagnostic Radiology. Lectures given to the Workman’s Compensation Board and Siemens MRI Symposium. Learning Objectives 1. Better understand Bone and Soft Tissue Tumors through individual cases. 2. Assess Internal Derangement of Joints through individual cases. 3. Improve your CanMEDS roles as a Scholar and Collaborator CASES 19 year old male Foot pain while running 1 Axial STIR Axial T1 Sagittal T1 22 year old male 2 months of elbow pain 2 Sagittal T1 Sagittal PD FS Coronal STIR Sagittal CT Recon Axial CT 9 year old male Growing ankle mass 3 Sagittal STIR Sagittal T1 AXIAL T2 52 year old female Slowly growing painless lump in triceps 4 Coronal T1 Coronal STIR COR T1 FS + GAD Ultrasound CASE REVIEW Case 1: DIFFUSE PIGMENTED VILLONODULAR SYNOVITIS (PVNS) OF CALCANEOCUBOID JOINT 1 Axial STIR Axial T1 Sagittal T1 1 Key Points NOTABLE: Mono-articular benign neoplasm, which is treated by synovectomy, but high-recurrence rates (50%) CLASSIC DESCRIPTOR: Low T1 and T2 lobulated tissue throughout the joint, with blooming on the gradient sequences due to hemosiderin PEARL: Almost never calcifies Cystic bone changes are usually in small joints Preserved joint space until late Case 1: PVNS of Calcaneocuboid Joint Companion Case – PVNS Wrist Coronal T2 Coronal GRE T2 Coronal T1 1 Differential Diagnosis 1. Amyloid Arthropathy a. Multiple joints (Systemic) 2. Chronic Hemorrhagic Effusions a. Rheumatoid arthritis b. Hemophilia c. Intra-articular Hemangioma, Sarcoma (very rare) 3. Synovial Chondromatosis a. Round or ovoid lesion, usually with calcification 4. -
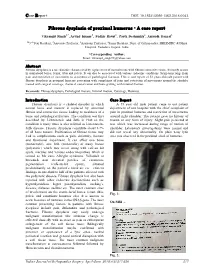
Fibrous Dysplasia of Proximal Humerus - a Case Report
Case Report DOI: 10.18231/2395-1362.2018.0043 Fibrous dysplasia of proximal humerus - A case report Vikramjit Singh1,*, Arvind Kumar2, Sudhir Rawat3, Parth Deshmukh4, Anirudh Bansal5 1,53rd Year Resident, 2Associate Professor, 3Assistant Professor, 4Senior Resident, Dept. of Orthopaedics, SBKSMIRC & Dhiraj Hospital, Vadodara, Gujarat, India *Corresponding Author: Email: [email protected] Abstract Fibrous dysplasia is a rare disorder characterized by replacement of normal tissue with fibrous connective tissue. It mostly occurs in craniofacial bones, femur, tibia and pelvis. It can also be associated with various endocrine conditions. Symptoms range from pain and restriction of movements to occurrence of pathological fractures. This is case report of 52 years old male patient with fibrous dysplasia in proximal humerus presenting with complaints of pain and restriction of movements around the shoulder treated with surgical curettage, chemical cauterization and bone grafting with internal fixation. Keywords: Fibrous dysplasia, Pathological fracture, Internal fixation, Curettage, Humerus. Introduction Case Report Fibrous dysplasia is a skeletal disorder in which A 55 year old male patient came to out patient normal bone and marrow is replaced by abnormal department of our hospital with the chief complaint of fibrous and connective tissue leading to weakness of a pain in proximal humerus and restriction of movements bone and pathological fracture. The condition was first around right shoulder. The patient gave no history of described by Lichtenstein and Jaffe in 1942 so the trauma or any form of injury. Slight pain persisted at condition is many times is also referred as Lictentstein- rest which was increased during range of motion of Jaffe disease. -
Bone and Soft Tissue Tumors
3/30/2009 BONE AND SOFT TISSUE TUMORS Fabrizio Remotti MD DEFINITION • Soft tissue pathology deals with tumors of the connective tissues. • The concept of soft tissue is understood broadly to include non-osseous tumors of extremities, trunk wall, retroperitoneum and mediastinum, and head & neck. • Excluded (with a few exceptions) are organ specific tumors. 1 3/30/2009 DEFINITION • Bone pathology deals with tumors of the skeletal system. • Included are subsets of tumors from extra- osseous sites that show osseous and cartilaginous differentiation. CLASSIFICATION • Purpose of classification is to link similar tumors in order to understand their behavior, determine the most approp riate treatment, and investi gate their biology. • However, purpose of a classification system is simplicity and reproducibility • Therefore tumors are classified according to the cell type they resemble. • Refinements are coming from cytogenetics, molecular, and gene expression studies. • The majority arise from -or show differentiation toward- mesenchymal cells, but some show other differentiation (neuroectodermal, histiocytic). • A small subset is of unknown histogenesis. 2 3/30/2009 CLASSIFICATION • Many tumors resemble tissues present in the region of origin. • These tumors may be derived from stem cells that belong to local, organ-specific pools. Vascular leiomyosarcoma • Other involved stem cells may be bone marrow derived. Lipoma Alveolar soft part sarcoma CLASSIFICATION • Some tumors have no resemblance to normal tissue in the region (metaplastic foci within a tumor, or tumors of different histogenesis from the normal cells of the region) • Some sarcomas have no normal cell counterparts, probably reflecting an unique genetic makeup. Epithelioid sarcoma, proximal type 3 3/30/2009 CLASSIFICATION • Tumors are also classified according their bio log ic po ten tia l. -

Systematic Approach of Sclerotic Bone Lesions Basis on Imaging Findings1 골경화성 병변에 대한 체계적 접근1
Pictorial Essay pISSN 1738-2637 / eISSN 2288-2928 J Korean Soc Radiol 2014;71(1):39-48 http://dx.doi.org/10.3348/jksr.2014.71.1.39 Systematic Approach of Sclerotic Bone Lesions Basis on Imaging Findings1 골경화성 병변에 대한 체계적 접근1 Se Kyoung Park, MD1, In Sook Lee, MD2, Kil Ho Cho, MD3, Jae Hyuck Yi, MD4, Sung Moon Lee, MD5, Sun Joo Lee, MD6, Jong Woon Song, MD7 1Department of Radiology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea 2Department of Radiology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea 3Department of Radiology, Yeungnam University Hospital, Daegu, Korea 4Department of Radiology, Kyungpook National University Hospital, Daegu, Korea 5Department of Radiology, Keimyung University Dongsan Medical Center, Keimyung University College of Medicine, Daegu, Korea 6Department of Radiology, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea 7Department of Radiology, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea Sclerotic bone lesions are common, but there are diverse groups of tumors and non-tumorous lesions. Although plain radiograph and computed tomography can Received February 21, 2014; Accepted May 9, 2014 reveal important characteristics of these lesions, diagnosis is often challenging for Corresponding author: In Sook Lee, MD Department of Radiology, Pusan National University radiologists. A systematic approach and familiarity with the imaging features of Hospital, Pusan National University School of Medicine, various sclerotic bone lesions may be greatly helpful for eliminating in the differen- 179 Gudeok-ro, Seo-gu, Busan 602-739, Korea. tial diagnosis. This review describes the systematic approach to diagnosing sclerotic Tel. -

Adamantinoma: a Clinicopathological Review and Update Deepali Jain*1, Vijay K Jain2, Rakesh K Vasishta3, Prabhat Ranjan3 and Yashwant Kumar3
Diagnostic Pathology BioMed Central Review Open Access Adamantinoma: A clinicopathological review and update Deepali Jain*1, Vijay K Jain2, Rakesh K Vasishta3, Prabhat Ranjan3 and Yashwant Kumar3 Address: 1Department of Pathology, Maulana Azad Medical College New Delhi, India, 2Department of Orthopedics Dr Ram Manohar Lohia Hospital, New Delhi, India and 3Department of Histopathology, Post Graduate Institute of Medical Education and Research, Chandigarh, India Email: Deepali Jain* - [email protected]; Vijay K Jain - [email protected]; Rakesh K Vasishta - [email protected]; Prabhat Ranjan - [email protected]; Yashwant Kumar - [email protected] * Corresponding author Published: 15 February 2008 Received: 1 October 2007 Accepted: 15 February 2008 Diagnostic Pathology 2008, 3:8 doi:10.1186/1746-1596-3-8 This article is available from: http://www.diagnosticpathology.org/content/3/1/8 © 2008 Jain et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Adamantinoma is a primary low-grade, malignant bone tumor that is predominantly located in the mid-portion of the tibia. The etiology of the tumor is still a matter of debate. The initial symptoms of adamantinoma are often indolent and nonspecific and depend on location and extent of the disease. Histologically, classic adamantinoma is a biphasic tumor characterized by epithelial and osteofibrous components that may be intermingled with each other in various proportions and differentiating patterns. To assure the histological diagnosis, pathologists should employ immunohistochemistry for demonstrating the sometimes sparse epithelial cell nests when the radiological features are suggestive of adamantinoma. -
BONE and SOFT TISSUE TUMORS SOFT TISSUE TUMORS Disorder Inheritance Locus Gene Tumor
CLASSIFICATION BONE AND SOFT TISSUE z Purpose of classification is to link similar tumors in order TUMORS to understand their behavior, determine the most appropriate treatment, and investigate their biology. z However, purpose of a classification system is simplicity and reproducibility z Therefore tumors are classified according to the cell type they resemble. z Refinements are coming from cytogenetics, molecular, and gene expression studies. z The majority arise from -or show differentiation toward- mesenchymal cells, but some show other differentiation (neuroectodermal, histiocytic). z A small subset is of unknown histogenesis. Fabrizio Remotti MD DEFINITION z Soft tissue pathology deals CLASSIFICATION with tumors of the connective tissues. z Many tumors resemble z The concept of soft tissue is tissues present in the understood broadly to region of origin. include non-osseous tumors z These tumors may be derived from stem cells of extremities, trunk wall, Vascular leiomyosarcoma retroperitoneum and that belong to local, mediastinum, and head & organ-specific pools. neck. z Other involved stem cells may be bone marrow z Excluded (with a few derived. exceptions) are organ specific tumors. Lipoma Alveolar soft part sarcoma DEFINITION CLASSIFICATION z Bone pathology deals with tumors of the z Some tumors have no skeletal system. resemblance to normal tissue in the region z Included are subsets of (metaplastic foci within a tumors from extra- ttftumor, or tumors of osseous sites that show different histogenesis from osseous and cartilaginous the normal cells of the region) differentiation. z Some sarcomas have no normal cell counterparts, probably reflecting an unique genetic makeup. Epithelioid sarcoma, proximal type 1 HISTOGENESIS EPIDEMIOLOGY All tumors are derived from stem cells that are programmed to differentiate into various mature cell types. -
Bone Tumours
Bone tumours Bone tumours are rare Improving the understanding of primary bone cancer Difficult for pathologists: considerable morphological overlap Subtypes differ in clinical behaviour and treatment Judith VMG Bovée Dept of Pathology In bone tumours: role of immunohistochemistry and LUMC molecular analysis is limited 1Insert > Header & footer 29-Apr-21 Diagnosis of bone tumours: multidisciplinary Diagnosis of bone tumours: multidisciplinary Classification of bone tumours WHO classification 2020 Three groups: 1. Soft tissue tumours 2. Undifferentiated Small Round Cell sarcomas of bone and soft tissue Expert board Four biological categories: benign intermediate locally aggressive intermediate rarely metastasizing malignant 1 Evaluation by an expert pathologist is crucial Centres of Expertise • Topreferral care • NFU: centre of expertise • EU: european reference network Patient Care 349 cases; 73% concordance 1463 patients, systematic review, 56% full 16% minor discrepancy concordance 35% partial concordance (different grade or Scientific 11% major discrepancy (5% benign / Innovation Research malignant) leading to management subtype) change 8% complete discordance (different diagnosis) Sarcoma 2009 Ann Oncol 2012 Netherlands Bone tumor committee Classification of Bone Tumours (WHO 2020) Netherlands Committee on Bone Tumours (since 1953) www.beentumoren.nl Netherlands committee for Bone Tumours secretariaat CvB dept. radiology LUMC consultation cases incomplete cases complete cases Benign n=20 clinical information clinical information radiology -

<I>Macaca Fascicularis</I>
Journal of the American Association for Laboratory Animal Science Vol 50, No 1 Copyright 2011 January 2011 by the American Association for Laboratory Animal Science Pages 98–104 Peripheral Ossifying Fibroma and Juxtacortical Chondrosarcoma in Cynomolgus Monkeys (Macaca fascicularis) Barthel Schmelting,1,* Martina Zöller,2,† and Joachim Kaspareit2 Literature on spontaneous primary bone tumors in nonhuman primates is sparse. This case report describes 2 different neoplastic bone lesions in 2 adult cynomolgus monkeys (Macaca fascicularis), including macroscopic, radiographic, histologic, and immunohistochemical findings. In one monkey, a firm mass located at the palatogingival junction of the left rostral max- illa was confirmed to be a peripheral ossifying fibroma in light of its histologic and immunohistochemical characteristics. In another monkey, a lobulated tumor at the right distal femur that radiographically showed moderate radiopacity with splotchy areas of mineralization was confirmed to be a juxtacortical chondrosarcoma on histologic examination. The 2 neoplastic bone lesions revealed rare histologic and immunohistochemical characteristics and contribute to the known tumor spectrum of cynomolgus monkeys. Spontaneous primary bone tumors are rare in nonhuman with objects for environmental enrichment, including movable primates.23 Only few references can be found on osteomas, stainless steel mirrors, colored plastic tools, and colored plastic osteosarcomas, and osteogenic sarcomas in various nonhuman balls. The bottom of the cages was enriched with wooden chips. primate species, including rhesus macaques (Macaca mulatta),1 A specialized primate diet (Sniff Pri 10 mm; sSniff Spezialdiäten squirrel monkeys (Saimiri sciureus),15,26 stumptail monkeys GmbH, Soest, Germany) was offered twice daily. The monkeys (Macaca arctoides),4 Japanese monkeys (Macaca fuscata),34 and regularly received fresh fruit and bread as food supplements. -

Benign Bone Tumors
Benign Bone Tumors Kambiz Motamedi, MD*, Leanne L. Seeger, MD KEYWORDS Benign bone tumor Radiography Computed tomography Magnetic resonance imaging The imaging characteristics of benign bone tumors signal focus on MR imaging on all pulse sequences reflect their histopathologic appearance. A solid with a normal surrounding marrow signal (Fig. 1). knowledge of this underlying histopathology aids Enostosis is usually inactive on bone scintigraphy. in differential diagnoses of these tumors. Important Further work-up is rarely necessary and biopsy is factors in diagnosis of a bone tumor include patient generally not indicated. A bone island may slightly age and gender; the bone involved; the location of fluctuate in size because of intrinsic osteoblastic or the tumor along, within, or on the bone; lesion osteoclastic activity, but a growth of more than margin; matrix proliferation; and periosteal reac- 25% over 6 months is unusual and may warrant tion. This article provides a review of the origin of a biopsy. The differential diagnosis includes sclerotic the tumor matrix and its influence on the imaging metastasis, but a metastatic lesion often demon- properties of these tumors. strates increased activity on bone scintigraphy and displays a halo of surrounding bone marrow edema 3 TUMORS WITH AN OSTEOID MATRIX on MR imaging on fluid-sensitive sequences. Multiple bone islands may be associated with These tumors contain a matrix derived from the osteopoikilosis (osteopathia disseminata); striped bone and cartilage progenitor cells of the embry- osteopathy (Voorhoeve disease); or melorheosto- onal mesenchyme. They encompass only 3.6% sis. Osteopoikilosis consists of numerous circular of biopsied bone tumors. or ovoid bone islands, usually on both sides of joints, whereas striped osteopathy is character- Enostosis ized by bandlike sclerotic foci in the bones. -

Benign Bone Tumor
BENIGN BONE TUMOR Prevalence of benign bone tumors. Osteochondroma 45% Osteoid Osteoma 10% Enchondroma 10% Haemangioma 10% Nonossifying fibroma 4% Osteoblastoma 2% Chondroblastoma 2% Chrondromyxoid fibroma 2% Clinical feature Pain: Local, Synovitis, Painful scoliosis Mass Deformity Pathological fracture Incidental finding Enneking System I Latent II. Active III. Aggressive Stage I Non Ossifying Fibroma Discovered incidentally Enchondroma Do not progress. Simple bone cyst May spontaneously resolve. Fibrous Dysplasia Need : observation alone. Osteochondroma When surgery: Intralesional excision Eosinophilic granuloma Stage II These lesions expand the host bone Simple bone cyst, Enchondroma They may destroy the cortex Osteoid osteoma Chrondromyxoid fibroma Surgery: intralesional curettage. Osteofibrous dysplasia Stage III Giant cell tumor Benign but aggressive tumor Osteoblastoma Chondroblastoma Soft tissue involvement Aneurysmal bone cyst. Present with pathologic fracture. Surgery: en bloc resection X ray 1. Where is the lesion Epiphyses: Chondroblastoma Giant cell tumor Diaphyses: Osteoid Osteoma Eosinophilic Granuloma Adamantinoma Metaphyses: Aneurysmal bone cyst Simple Bone Cyst Non ossifying fibroma Chrondromyxoid fibroma 1. SIMPLE BONE CYST Simple bone cysts occur in young children [6‐14 years] Common site: Proximal humerus and proximal femur Usually metaphyseal. With age, it can move towards the diaphyses Radiological Osteolytic Metaphyseal Centric With or without pathological No periosteal reaction [when no pathological fracture] When fractured: fallen leaf sign. Treatment 1.Proximal humerus: The current standard of care is the injection of corticosteroid 2. For proximal femur: Curettage, bone graft and hip screw and plate Companacci: Steroid Vs surgery (170 in each group) 42% healed with one injection and 96% with multiple injection. With bone grafting 46% healed. 2. ANEURYSMAL BONE CYST 75% patients are under 20 years.