November 28, 2017 Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Dance Division Calendar 17-18
Early Dance Division 2017-2018 Session 1 September 9 – November 3 Monday Classes Tuesday Classes September 11 Class September 12 Class September 18 Class September 19 Class September 25 Class September 26 Class October 2 Class October 3 Class October 9 Class October 10 Class October 16 Class October 17 Class October 23 Class October 24 Class October 30 Last Class October 31 Last Class Wednesday Classes Thursday Classes September 13 Class September 14 Class September 20 Class September 21* Class September 27 Class September 28 Class October 4 Class October 5 Class October 11 Class October 12 Class October 18 Class October 19 Class October 25 Class October 26 Class November 1 Last Class November 2 Last Class Saturday Classes Sunday Classes September 9 Class September 10 Class September 16 Class September 17 Class September 23 Class September 24 Class September 30* Class October 1 Class October 7 Class October 8 Class October 14 Class October 15 Class October 21 Class October 22 Class October 28 Last Class October 29 Last Class *Absences due to the holiday will be granted an additional make-up class. Early Dance Division 2017-2018 Session 2 November 4 – January 22 Monday Classes Tuesday Classes November 6 Class November 7 Class November 13 Class November 14 Class November 20 No Class November 21 No Class November 27 Class November 28 Class December 4 Class December 5 Class December 11 Class December 12 Class December 18 Class December 19 Class December 25 No Class December 26 No Class January 1 No Class January 2 No Class January 8 Class -

Academic Calendar
Academic Calendar 2018 – 2019 Academic Year Dallas Fall 2018: August 27, 2018 – December 16, 2018 EVENT DATE NOTES First Day of Instruction August 27, 2018 Week 1 of Fall Last Day to Add Courses September 2, 2018 End of Week 1 Labor Day September 3, 2018 Holiday Last Day to Drop with a “W” October 21, 2018 End of Week 8 Spring 2019 Schedule Preview October 22, 2018 Spring 2019 Registration October 29, 2018 Veterans Day (Observed) November 12, 2018 Holiday Thanksgiving Holiday November 22 - 23, 2018 Holiday Last Day of Instruction December 3, 2018 Week 15 Grades Due December 11, 2018 Official End of Semester December 16, 2018 Spring 2019: January 7, 2019 – April 28, 2019 First Day of Instruction January 7, 2019 Week 1 of Spring Last Day to Add Courses January 13, 2019 End of Week 1 Martin Luther King Jr Day January 21, 2019 Holiday Last Day to Drop with a “W” March 3, 2019 End of Week 8 Summer 2019 Schedule Preview March 4, 2019 Summer 2019 Registration March 11, 2019 Last Day of Instruction April 15, 2019 Week 15 Grades Due April 23, 2019 Official End of Semester April 28, 2019 Summer 2019: May 6, 2019 – August 25, 2019 All Programs: 8 Week Courses First Day of Instruction May 6, 2019 Week 1 of Summer Last Day to Add Courses May 12, 2019 End of Week 1 Memorial Day May 27, 2019 Holiday Fall 2019 Schedule Preview June 3, 2019 Last Day to Drop with a “W” June 9, 2019 End of Week 5 Fall 2019 Registration Begins June 10, 2019 Last Day of Instruction June 30, 2019 End of Week 8 Independence Day July 4, 2019 Holiday Grades Due July 8, 2019 Official End of Semester August 25, 2019 Dates subject to change. -

Division of Personnel Employee Information and Payroll Audit Section
Division of Personnel Employee Information and Payroll Audit Section This document is provided as a service to state agencies. Note: The 90-day interval includes both the beginning and ending dates. 90-DAY INTERVAL CALENDAR FOR 2011 JANUARY 2011 If the date is... 90th weekday after the date is... JANUARY 3 MONDAY MAY 6 FRIDAY JANUARY 4 TUESDAY MAY 9 MONDAY JANUARY 5 WEDNESDAY MAY 10 TUESDAY JANUARY 6 THURSDAY MAY 11 WEDNESDAY JANUARY 7 FRIDAY MAY 12 THURSDAY JANUARY 10 MONDAY MAY 13 FRIDAY JANUARY 11 TUESDAY MAY 16 MONDAY JANUARY 12 WEDNESDAY MAY 17 TUESDAY JANUARY 13 THURSDAY MAY 18 WEDNESDAY JANUARY 14 FRIDAY MAY 19 THURSDAY JANUARY 17 MONDAY MAY 20 FRIDAY JANUARY 18 TUESDAY MAY 23 MONDAY JANUARY 19 WEDNESDAY MAY 24 TUESDAY JANUARY 20 THURSDAY MAY 25 WEDNESDAY JANUARY 21 FRIDAY MAY 26 THURSDAY JANUARY 24 MONDAY MAY 27 FRIDAY JANUARY 25 TUESDAY MAY 30 MONDAY JANUARY 26 WEDNESDAY MAY 31 TUESDAY JANUARY 27 THURSDAY JUNE 1 '11 WEDNESDAY JANUARY 28 FRIDAY JUNE 2 THURSDAY JANUARY 31 MONDAY JUNE 3 FRIDAY FEBRUARY 2011 If the date is... 90th weekday after the date is... FEBRUARY 1 TUESDAY JUNE 6 MONDAY FEBRUARY 2 WEDNESDAY JUNE 7 TUESDAY FEBRUARY 3 THURSDAY JUNE 8 WEDNESDAY FEBRUARY 4 FRIDAY JUNE 9 THURSDAY FEBRUARY 7 MONDAY JUNE 10 FRIDAY FEBRUARY 8 TUESDAY JUNE 13 MONDAY FEBRUARY 9 WEDNESDAY JUNE 14 TUESDAY FEBRUARY 10 THURSDAY JUNE 15 WEDNESDAY FEBRUARY 11 FRIDAY JUNE 16 THURSDAY FEBRUARY 14 MONDAY JUNE 17 FRIDAY FEBRUARY 15 TUESDAY JUNE 20 MONDAY FEBRUARY 16 WEDNESDAY JUNE 21 TUESDAY FEBRUARY 17 THURSDAY JUNE 22 WEDNESDAY FEBRUARY 18 FRIDAY JUNE 23 THURSDAY FEBRUARY 21 MONDAY JUNE 24 FRIDAY FEBRUARY 22 TUESDAY JUNE 27 MONDAY FEBRUARY 23 WEDNESDAY JUNE 28 TUESDAY FEBRUARY 24 THURSDAY JUNE 29 WEDNESDAY FEBRUARY 25 FRIDAY JUNE 30 THURSDAY FEBRUARY 28 MONDAY JULY 1 '11 FRIDAY MARCH 2011 If the date is.. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
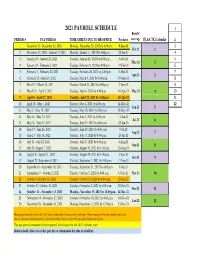
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -

Thanksgiving Weather Louisville, Kentucky
Thanksgiving Weather Louisville, Kentucky Highest temperature: 73° on November 26, 1896 Highest daily average temperature: 65.5° on November 26, 1896 Lowest temperature: 8° on November 27, 1930 Lowest average daily temperature: 14.5° on November 27, 1930 Wettest: 2.19” on November 25, 2010 Snowiest: 1.2” on November 24, 1938 Deepest Snow Cover: 1” on November 28, 1929; November 27, 1930; and November 25, 1971 High Temp Low Temp Precipitation Snowfall Snow Depth November 28, 1872 39 19 0 November 27, 1873 49 34 0 November 26, 1874 46 25 0 November 25, 1875 52 27 0.05 November 30, 1876 33 24 0.03 November 29, 1877 32 23 0 November 28, 1878 45 36 0 November 27, 1879 65 56 1 November 25, 1880 31 24 0.23 November 24, 1881 27 19 T November 30, 1882 42 32 0 November 29, 1883 56 37 0 November 27, 1884 60 45 0 0 November 26, 1885 49 38 0 0 November 25, 1886 39 25 T T November 24, 1887 60 48 0.32 0 November 29, 1888 42 37 0.01 0 November 28, 1889 37 23 0.06 0.1 November 27, 1890 51 27 0 0 November 26, 1891 52 37 T 0 November 24, 1892 38 16 0 0 November 30, 1893 49 38 0 0 November 29, 1894 40 23 T 0 November 28, 1895 55 28 0 0 November 26, 1896 73 58 0 0 November 25, 1897 56 47 0.23 0 November 24, 1898 35 21 0 0 November 30, 1899 66 48 0 0 November 29, 1900 39 31 0 0 0 High Temp Low Temp Precipitation Snowfall Snow Depth November 28, 1901 42 27 0 0 0 November 27, 1902 37 32 T T 0 November 26, 1903 29 19 0 0 0 November 24, 1904 66 35 0 0 0 November 30, 1905 30 22 0 0 0 November 29, 1906 43 32 0 0 0 November 28, 1907 51 36 0 0 0 November 26, 1908 -
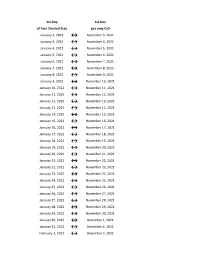
Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 2, 2022 ↔ November 3, 2021 January 3, 2022 ↔ November 4, 2021 January 4, 2022 ↔ November 5, 2021 January 5, 2022 ↔ November 6, 2021 January 6, 2022 ↔ November 7, 2021 January 7, 2022 ↔ November 8, 2021 January 8, 2022 ↔ November 9, 2021 January 9, 2022 ↔ November 10, 2021 January 10, 2022 ↔ November 11, 2021 January 11, 2022 ↔ November 12, 2021 January 12, 2022 ↔ November 13, 2021 January 13, 2022 ↔ November 14, 2021 January 14, 2022 ↔ November 15, 2021 January 15, 2022 ↔ November 16, 2021 January 16, 2022 ↔ November 17, 2021 January 17, 2022 ↔ November 18, 2021 January 18, 2022 ↔ November 19, 2021 January 19, 2022 ↔ November 20, 2021 January 20, 2022 ↔ November 21, 2021 January 21, 2022 ↔ November 22, 2021 January 22, 2022 ↔ November 23, 2021 January 23, 2022 ↔ November 24, 2021 January 24, 2022 ↔ November 25, 2021 January 25, 2022 ↔ November 26, 2021 January 26, 2022 ↔ November 27, 2021 January 27, 2022 ↔ November 28, 2021 January 28, 2022 ↔ November 29, 2021 January 29, 2022 ↔ November 30, 2021 January 30, 2022 ↔ December 1, 2021 January 31, 2022 ↔ December 2, 2021 February 1, 2022 ↔ December 3, 2021 1st Day 1st Day of Your Desired Stay you may Call February 2, 2022 ↔ December 4, 2021 February 3, 2022 ↔ December 5, 2021 February 4, 2022 ↔ December 6, 2021 February 5, 2022 ↔ December 7, 2021 February 6, 2022 ↔ December 8, 2021 February 7, 2022 ↔ December 9, 2021 February 8, 2022 ↔ December 10, 2021 February 9, 2022 ↔ December 11, 2021 February 10, 2022 ↔ December 12, 2021 February -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

20-21 MHS FALL CALENDAR.Pdf
Monterey High School Monterey High School st nd Fall 1 Quarter: 8/17/20-10/16/20 Fall 2 Quarter: 10/19/20 – 12/18/20 SUNDAY MONDAY TUESDAY WEDNESDAY THURSDAY FRIDAY SATURDAY SUNDAY MONDAY TUESDAY WEDNESDAY THURSDAY FRIDIAY SATURDAY August 2 August 3 August 4 August 5 August 6 August 7 August 8 August 9 August 10 August 11 August 12 August 13 August 14 August 15 District Staff District Staff District Staff Teacher Teacher Development Development Development Work Day Work Day August 16 August 17 August 18 August 19 August 20 August 21 August 22 October 18 October 19 October 20 October 21 October 22 October 23 October 24 Instruction Start New Qtr Begins August 23 August 24 August 25 August 26 August 27 August 28 August 29 October 25 October 26 October 27 October 28 October 29 October 30 October 31 August 30 August 31 September 1 September 2 September 3 September 4 Sept 5 November 1 November 2 November 3 November 4 November 5 November 6 November 7 Sept 6 September 7 September 8 September 9 September September 11 Sept 12 November 8 November 9 November 10 November 11 November November 13 November 14 10 12 Labor Day Back to Veteran’s School Night Day Sept 13 September 14 September 15 September 16 September September 18 Sept 19 November 15 November 16 November 17 November 18 November November 20 November 21 17 19 September 21 September 22 September 23 September September 25 Sept 26 November 22 November 23 November 24 November 25 November November 27 November 28 24 26 NO SCHOOL Thanksgiving No School Sept 27 September 28 September 29 September -

COVID-19 Dashboard
11/28/2020 Public Health Indicators Massachusetts Department of Public Health COVID-19 Dashboard- Saturday, November 28, 2020 Dashboard of Public Health Indicators Total Cases By Age Group Testing and Cases from 11/8/2020-11/21/2020 * Newly Reported Total New 7-Day Average Estimated Active Confirmed Cases Molecular Tests Positivity Cases 0-19 years 5,924 Reported 2,914 72,269 3.57% 42,549 20-29 years 7,051 Hospitalization 30-39 years 5,740 Total Confirmed Total Confirmed Average Age of COVID Patients in COVID Patients in Cases that were 40-49 years 4,633 Hospital ICU Hospitalized* 1,045 225 65 50-59 years 4,803 Deaths Turnaround Time 60-69 years 3,198 Newly Reported Average Age of 14-Day Average Turnaround (from Test Deaths among Deaths* Sample to Report to DPH)* 70-79 years 1,544 Confirmed 40 81 1.92 80+ years 1,113 Note: For definitions, please see the Glossary at the end of this document in "Definitions and Disclaimers". Average age of hospitalized cases and deaths are calculated for a two week period covering 11/08/2020 to 11/21/2020 . Please see the most current weekly dashboard for more details https://www.mass.gov/info-details/covid-19-response-reporting *Last updated Wednesday, November 25, 2020 1 1/1 11/28/2020 Public Health Indicators2 Massachusetts Department of Public Health COVID-19 Dashboard- Saturday, November 28, 2020 Percent of Change Since Dashboard of Public Health Indicators Lowest Observed Value (LOV) 7-Day Average of 3,000 2,588 2,451 2,499 2,541 2,575 2,574 2,549 2,461 2,307 2,360 2,415 2,403 New Confirmed 2,154