Clinically Isolated Neurosarcoidosis: a Recommended Diagnostic Path
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Consequences of Sarcoidosis
Consequences of Sarcoidosis Marjolein Drent, MD, PhDa,b,c,*, Bert Strookappe, MScc,d, Elske Hoitsma, MD, PhDc,e, Jolanda De Vries, MSc, PhDc,f,g KEYWORDS Cognitive impairment Depressive symptoms Exercise limitation Fatigue Pain Rehabilitation Sarcoidosis Small fiber neuropathy Quality of life KEY POINTS Consequences of sarcoidosis are wide ranging, and have a great impact on patients’ lives. Sarcoidosis patients suffer not only from organ-related symptoms, but also from a wide spectrum of rather nonspecific disabling symptoms. Absence of evidence does not mean evidence of absence. Management of sarcoidosis requires a multidisciplinary personalized approach that focuses on somatic as well as psychosocial aspects of the disease. INTRODUCTION of sarcoidosis patients include symptoms that cannot be explained by granulomatous involve- The clinical expression, natural history, and prog- ment of a particular organ.4 Apart from lung- nosis of sarcoidosis are highly variable and its related symptoms (eg, coughing, breathlessness, course is often unpredictable.1 Clinical manifesta- 1,2 and dyspnea on exertion), patients may suffer tions vary with the organs involved. The lungs from a wide range of rather nonspecific disabling are affected in approximately 90% of patients symptoms.2,5 These symptoms, such as fatigue, with sarcoidosis, and the disease frequently also fever, anorexia, arthralgia, muscle pain, general involves the lymph nodes, skin, and eyes. Remis- weakness, muscle weakness, exercise limitation, sion occurs in more than one-half of patients within and cognitive failure, often do not correspond 3 years of diagnosis, and within 10 years in two- 2,5–9 2 with objective physical evidence of disease. thirds, with few or no remaining consequences. -

An Elderly Patient with Sarcoidosis Manifesting Panhypopituitarism with Central Diabetes Insipidus
Endocrine Journal 2007, 54 (3), 425–430 An Elderly Patient with Sarcoidosis Manifesting Panhypopituitarism with Central Diabetes Insipidus TOMOKO MIYOSHI, FUMIO OTSUKA, MASAYA TAKEDA, KENICHI INAGAKI, HIROYUKI OTANI, TOSHIO OGURA, KEN ICHIKI*, TETSUKI AMANO* AND HIROFUMI MAKINO Department of Medicine and Clinical Science, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Okayama City, 700-8558, Japan *Aioi City Hospital, 5-12 Sakae-cho, Aioi City, 678-0008, Japan Abstract. We here report a 77-year-old Japanese male who suffered general fatigue with progressive thirst and polyuria. Central diabetes insipidus was diagnosed by depletion of vasopressin secretion in response to increases in serum osmolality. Secretory responses of anterior pituitary hormones including adrenocorticotropin, thyrotropin, gonadotropins and growth hormone were severely impaired. Diffuse swelling of the infundibulum as well as lack of T1-hyperintense signal in the posterior lobe was noted by pituitary magnetic resonance imaging. The presence of bilateral hilar lymphade- nopathy and increased CD4/CD8 ratio in bronchoalveolar lavage fluid was diagnostic for lung sarcoidosis. Physiological doses of corticosteroid and thyroid hormone were administered in addition to desmopressin supplementation. Complete regression of the neurohypophysial swelling was notable two years after corticosteroid replacement. Diffuse damage of anterior pituitary combined with hypothalamic involvement leading to central diabetes insipidus is a rare manifestation in such elderly patients with neurosarcoidosis. Key words: Central diabetes insipidus, Hypophysitis, Lymphocytic infundibuloneurohypophysitis, Neurosarcoidosis, Panhypopituitarism, Sarcoidosis (Endocrine Journal 54: 425–430, 2007) SARCOIDOSIS is a systemic granulomatous disease dysfunction and less frequently involve the infundibu- involving multiple organs, in which endocrinopathy is lum and/or the pituitary gland, leading to hypothalamic rarely complicated [1]. -

Coexistence of Neurosarcoidosis and Multiple Sclerosis
Neurol. Croat. Vol. 61, 3-4, 2012 Coexistence of neurosarcoidosis and multiple sclerosis L. Radolović Prenc1, S.Telarović2,3, I.Vidović1, J. Sepčić4, L. Labinac Peteh1 ABSTRACT - Sarcoidosis is a multisystem infl ammatory disease of unknown etiology that predominantly aff ects the lungs and intrathoracic lymph nodes, but in 6% of cases it also occurs in central or peripheral nervous system. Multiple sclerosis (MS) is an immune-mediated infl ammatory disease that attacks myeli- nated axons in the central nervous system. Coexistence of sarcoidosis and other autoimmune diseases like MS is rarely reported in the literature. We present a case report of a patient with coexisting sarcoidosis and 67 MS, with a positive family history of MS. Symptoms of sarcoidosis appeared three years before the onset of Number 3-4, 2012 Number symptoms typical for MS. Similarity of demyelinating lesions in the nervous system, increased IgG in cere- brospinal fl uid and good response to corticosteroid treatment point to similar etiology. Th e onset of diseases like sarcoidosis and MS in the same patient over a period of only a few years opens the question whether the two separate entities come in sequence or the onset of sarcoidosis occurs during development of typical clinical presentation of MS. Key words: sarcoidosis, encephalomyelitis, multiple sclerosis INTRODUCTION lymph nodes (Figs. 1 and 2), or pathologic skin nodes or bone cysts, especially in hands, and posi- Sarcoidosis is an infl ammatory disorder of un- tive Kveim test (2). Cerebrospinal fl uid (CSF) anal- known origin, characterized by epithelioid cell ysis shows increased IgG, pleocytosis with protein- granulomas in various organs (1). -

Neurosarcoidosis
CHAPTER 11 Neurosarcoidosis E. Hoitsma*,#, O.P. Sharma} *Dept of Neurology and #Sarcoidosis Management Centre, University Hospital Maastricht, Maastricht, The Netherlands, and }Dept of Pulmonary and Critical Care Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA. Correspondence: O.P. Sharma, Room 11-900, LACzUSC Medical Center, 1200 North State Street, Los Angeles, CA 90033, USA. Fax: 1 3232262728; E-mail: [email protected] Sarcoidosis is an inflammatory multisystemic disorder. Its cause is not known. The disease may involve any part of the nervous system. The incidence of clinical involvement of the nervous system in a sarcoidosis population is estimated to be y5–15% [1, 2]. However, the incidence of subclinical neurosarcoidosis may be much higher [3, 4]. Necropsy studies suggest that ante mortem diagnosis is made in only 50% of patients with nervous system involvement [5]. As neurosarcoidosis may manifest itself in many different ways, diagnosis may be complicated [2, 3, 6–10]. It may appear in an acute explosive fashion or as a slow chronic illness. Furthermore, any part of the nervous system can be attacked by sarcoidosis, but the cranial nerves, hypothalamus and pituitary gland are more commonly involved [1]. Sarcoid granulomas can affect the meninges, parenchyma of the brain, hypothalamus, brainstem, subependymal layer of the ventricular system, choroid plexuses and peripheral nerves, and also the blood vessels supplying the nervous structures [11, 12]. One-third of neurosarcoidosis patients show multiple neurological lesions. If neurological syndromes develop in a patient with biopsy- proven active systemic sarcoidosis, the diagnosis is usually easy. However, without biopsy evidence of sarcoidosis at other sites, nervous system sarcoidosis remains a difficult diagnosis [13]. -

The Pathogenesis and Treatment of Optic Disc Swelling in Neurosarcoidosis a Unique Therapeutic Response to Infliximab
OBSERVATION The Pathogenesis and Treatment of Optic Disc Swelling in Neurosarcoidosis A Unique Therapeutic Response to Infliximab Jeffrey M. Katz, MD; Michiko Kimura Bruno, MD; Jacqueline M. S. Winterkorn, MD, PhD; Nancy Nealon, MD Objective: To review the pathogenesis and treatment eye. A 57-year-old woman presented with bilateral, sub- of optic disc swelling in neurosarcoidosis, including a acute, painful visual loss and unilateral papillitis consis- novel therapeutic response to infliximab. tent with optic neuritis. Her visual loss responded rap- idly to intravenous corticosteroids. The funduscopic Design and Setting: Case reports from an inpatient examination findings in both patients prompted further neurology service. clinical investigation, culminating in the diagnosis of neu- rosarcoidosis. Patients: A 35-year-old woman presented with head- ache, chronic visual loss, papilledema, and optic atro- Conclusion: Understanding the multiple etiologic mecha- phy, characteristic of chronic intracranial hypertension. nisms that produce optic disc swelling in sarcoidosis can Magnetic resonance imaging showed bifrontal cerebral help neurologists tailor treatment for patients with neu- edema with en plaque frontal pachymeningeal enhance- rosarcoidosis who present with this symptom. ment. Her visual loss progressed despite conventional therapies. The use of the tumor necrosis factor ␣ antago- nist infliximab maintained functional vision in her right Arch Neurol. 2003;60:426-430 OSS OF VISION associated with 20/25 OD with a constricted visual field (VF) optic disc swelling (ODS) is and an inferonasal step. The left eye had no a rare initial presentation of light perception and an amaurotic pupil. neurosarcoidosis. Optic disc Funduscopic examination findings re- swelling is an important vealed right optic disc swelling (Figure 1A) Lclinical sign because it can herald central and left optic disc pallor and resolving swell- nervous system disease in an otherwise ing (Figure 1B). -

Sarcoidosis Optic Neuropathy
Sarcoidosis Optic Neuropathy Optometric Retina Society Residency Award Delia Groshek, OD White River Junction VA Medical Center Ocular Disease Residency 2010-2011 6244 North Knox Avenue Chicago, IL 60646 (773) 620-5993 [email protected] Abstract Sarcoidosis is a multi-systemic granulomatous disease of unknown etiology that can affect almost every organ in the body, though it typically affects the lung, lymph nodes, skin, liver and eyes. The diagnosis of sarcoidosis is based on histological evidence of noncaseating epithelioid cell granulomas, bilateral hilar lymphadenopathy, and exclusion of other diseases that produce a similar clinical and/or histological picture. Ocular involvement may be the presenting sign of the disease and can involve any ocular structure. In this case, a 67 year old Caucasian male presents with blur, photopsia, papillitis, and periphlebitis. This case report reviews the diagnosis, treatment and management of ocular sarcoidosis. Key words: angiotension converting enzyme, bilateral hilar lymphadenopathy, noncaseating granuloma, papillitis, periphlebitis, sarcoidosis Case Report A 67 year old Caucasian male presented to the VA eye clinic with a chief complaint of central blurring of his vision in his left eye, which happened twice the previous night lasting for five minutes each time. Afterwards, he saw blue spots. In the exam, he stated his left eye felt like it had a dull, aching pain of a 2-3 on a scale of 10. He reported itching and tearing of his left eye. Upon further questioning, the patient denied scalp tenderness, jaw claudication, or recent weight loss. The patient did report neck pain, which has been stable for years due to his arthritis. -

Neurosarcoidosis: Clinical Manifestations, Investigation and Treatment
REVIEW Pract Neurol: first published as 10.1136/practneurol-2019-002349 on 17 May 2020. Downloaded from Neurosarcoidosis: clinical manifestations, investigation and treatment Desmond P Kidd Centre for Neurosarcoidosis, ABSTRACT eye may be involved, and skin, cardiac, Neuroimmunology unit, Sarcoidosis affects the nervous system in 10% hepatic, renal, bone and joint involve- Institute of Immunology and 3 Transplantation, University of cases. When it does so it can affect any part ment is common. College London, London, UK of the nervous system and with all degrees Around 30% of cases resolve within of severity. It forms part of the differential 2 years, particularly with single system Correspondence to Dr Desmond P Kidd, Royal Free diagnosis in inflammatory, infective, neoplastic involvement, 30% have a relapsing form Hospital, London NW3 2QG, UK; and degenerative neurological diseases and and 30% progressively deteriorate. d. kidd@ ucl. ac. uk may be very difficult to diagnose without histological confirmation. Recent clinical studies Accepted 5 January 2020 Epidemiology and the increasing availability of new biological Differences in the prevalence of the treatments allow a much clearer understanding disease have been known for decades; it of the disease. This review summarises its clinical has long been known that the incidence features, imaging and laboratory characteristics, is highest in Nordic countries 11–24 treatment and outcome. per 105,4 and lowest in south- east Asian countries (0.85 in Korea, 2.17 in Taiwan and 1.01 in Japan).5–8 African Americans INTRODUCTION within the USA have three times the inci- Sarcoidosis is an auto-inflammatory dence of European Americans,9 and the disorder characterised by the develop- incidence is lower still in Hispanic Amer- ment of granulomatous inflammation in icans.10 Recent studies from the Swedish affected tissues. -

Neurosarcoidosis-Demonstration of Meningeal Disease by Gadolinium
Journal ofNeurology, Neurosurgery, and Psychiatry 199 1;54:499-502 499 Neurosarcoidosis-demonstration of meningeal J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.54.6.499 on 1 June 1991. Downloaded from disease by gadolinium enhanced magnetic resonance imaging K T Khaw, H Manji, J Britton, F Schon Abstract (attributed to bilateral mastoidectomies) and Arriving at a firm diagnosis of neuro- left sided ninth to twelfth lower cranial nerve sarcoidosis continues to pose serious palsies. Cerebrospinal fluid examination was problems, particularly when evidence of unremarkable as was myelography and ver- granulomatous disease outside the tebral angiography. Biopsies taken of a nervous system is lacking. The common- possible mass in the left nasopharynx revealed est mode of presentation of neuro- no definite abnormality. Her erythrocyte sarcoidosis is with cranial nerve palsies. sedimentation rate was 40 mm/hr. Her Two cases of presumed neurosarcoidosis neurological condition again spontaneously with cranial nerve palsies showed clear improved. She remained well until the age of evidence of focal meningeal disease on 61 when a 5 x 7 cm mass was removed from gadolinium-DTPA enhanced MRI brain the left buttock which on histological examin- scans. Although not specific for sar- ation was consistent with erythema nodosum. coidosis, this technique may be very The patient then developed vaginal bleeding useful in aiding the diagnosis in sus- and other persistent gynaecological symp- pected cases. toms. These were treated surgically with hys- terectomy and bilateral salpingo-oophorec- Sarcoidosis is a disseminated disease of un- tomy at the age of 63 and colpectomy at the known aetiology characterised by non-caseat- age of 64. -

Neurosarcoidosis Manifesting As Tremor of the Extremities and Severe Hypopituitarism —Case Report—
Neurol Med Chir (Tokyo) 48, 314¿317, 2008 Neurosarcoidosis Manifesting as Tremor of the Extremities and Severe Hypopituitarism —Case Report— Yoshikazu OGAWA,TeijiTOMINAGA*,andHidetoshiIKEDA** Department of Neurosurgery, Kohnan Hospital, Sendai, Miyagi; *Department of Neurosurgery, Tohoku Graduate School of Medicine, Sendai, Miyagi; **Department of Neurosurgery, Ohara Medical Center, Fukushima Abstract A 48-year-old woman initially presented with significant tremor of the extremities and subsequent severe hypopituitarism. Magnetic resonance imaging showed hyperintense areas in bilateral caudate heads and putamina, and a pituitary mass. L-dopa and corticosteroid were given and the tremor was reduced. Serum markers including autoimmune diseases were negative. Computed tomography and positron emission tomography detected no abnormalities except for pituitary lesion. Transsphenoidal biopsy revealed a noncaseating granuloma including giant cells with destroyed pituitary gland. The diagnosis was sarcoidosis. Diagnosis of isolated neurosarcoidosis is definitely difficult. Biopsy may be essential to establish the diagnosis in such a case. Corticosteroid administration is strongly recom- mended to avoid irreversible damage to the normal tissues even if histological confirmation was not achieved. Key words: basal ganglia, hypopituitarism, neurosarcoidosis, tremor Introduction Case Presentation Neurosarcoidosis accounts for about 5% of cases of A 48-year-old woman suffering from severe tremor systemic sarcoidosis.2) The major types of clinical of -
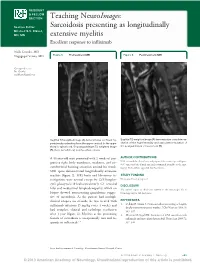
Sarcoidosis Presenting As Longitudinally Extensive
RESIDENT & FELLOW SECTION Teaching NeuroImages: Section Editor Sarcoidosis presenting as longitudinally Mitchell S.V. Elkind, MD, MS extensive myelitis Excellent response to infliximab Naila Goenka, MD Nagagopal Venna, MD Figure 1 Pretreatment MRI Figure 2 Posttreatment MRI Correspondence to Dr. Goenka: [email protected] Sagittal T2-weighted image (A) demonstrates confluent hy- Sagittal T2-weighted image (A) demonstrates complete res- perintensity extending from the upper cervical to the upper olution of the hyperintensity and associated resolution of thoracic spinal cord. On postgadolinium T1-weighted image the postgadolinium enhancement (B). (B), there is multifocal, patchy enhancement. A 44-year-old man presented with 2 weeks of pro- AUTHOR CONTRIBUTIONS gressive right body numbness, weakness, and cir- N.G. reviewed the clinical case and prepared the manuscript and figures. N.V. supervised the clinical care and commented critically on the man- cumferential burning sensation around his trunk. uscript. Both authors approved the final version. MRI spine demonstrated longitudinally extensive myelitis (figure 1). MRI brain and laboratory in- STUDY FUNDING vestigations were normal except for CSF lympho- No targeted funding reported. cytic pleocytosis (9 leukocytes/mm3). CT revealed DISCLOSURE hilar and mediastinal lymphadenopathy, which on The authors report no disclosures relevant to the manuscript. Go to biopsy showed noncaseating granulomas sugges- Neurology.org for full disclosures. tive of sarcoidosis. As the patient had multiple clinical relapses on steroids, he was treated with REFERENCES infliximab infusions (5 mg/kg every 4 weeks) and 1. Dolhun R, Sriram S. Neurosarcoidosis presenting as longitu- dinally extensive transverse myelitis. J Clin Neurosci 2009;16: had complete clinical and radiologic resolution 595–597. -
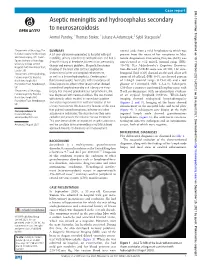
Aseptic Meningitis and Hydrocephalus Secondary to Neurosarcoidosis Anmol Pandey,1 Thomas Stoker,1 Lukasz a Adamczyk,2 Sybil Stacpoole3
Case report BMJ Case Rep: first published as 10.1136/bcr-2021-242312 on 26 August 2021. Downloaded from Aseptic meningitis and hydrocephalus secondary to neurosarcoidosis Anmol Pandey,1 Thomas Stoker,1 Lukasz A Adamczyk,2 Sybil Stacpoole3 1Department of Neurology, The SUMMARY normal aside from a mild lymphopaenia which was National Hospital for Neurology A 53- year- old woman presented to hospital with gait present from the onset of her symptoms in May. and Neurosurgery, UCL Queen instability, urinary incontinence and confusion. She had a Serum Angiotensin Converting Enzyme (ACE) was Square Institute of Neurology, 4- month history of headache, blurred vision, personality non- elevated at <12 units/L (normal range (NR): University College London change and memory problems. Magnetic Resonance 20–70). Her Addenbrooke’s Cognitive Examina- Hospitals NHS Foundation Trust, tion–Revised (ACE-R) score was 45/100. Her cere- London, UK Imaging of the brain after contrast application 2Department of Histopathology, showed tectal plate and occipital enhancement, brospinal fluid (CSF) showed an elevated white cell Peterborough City Hospital, as well as a known hydrocephalus. Cerebrospinal count of 60 cells/µL (NR: 0–5), an elevated protein North West Anglia NHS fluid showed aseptic meningitis with no evidence of of 1.04 g/L (normal range: 0.15–0.45) and a low Foundation Trust, Peterborough, clonal expansion. After further imaging that showed glucose of 1.6 mmol/L (NR: 2.2–4.0). Subsequent UK generalised lymphadenopathy and subsequent tissue CSF flow cytometry confirmed lymphocytosis with 3Department of Neurology, biopsy that showed granulomatous lymphadenitis, she T- cell predominance with no phenotypic evidence Peterborough City Hospital, was diagnosed with neurosarcoidosis. -

Sarcoidosis Treatment Guidelines
SARCOIDOSIS TREATMENT GUIDELINES INTRODUCTION Goals of Sarcoidosis Management Sarcoidosis is a chronic inflammatory granulomatous dis- The goals of sarcoidosis management are to prevent or con- ease that primarily affects the lungs, although multi-organ trol organ damage, relieve symptoms and improve the involvement is common. The etiology of sarcoidosis is not patient’s quality of life. An evaluation by a pulmonologist is clear; however, genetic and environmental factors probably strongly recommended. For patients with extrapulmonary play a role in the development and expression of the disease. involvement, a multidisciplinary approach may be required. A patient may need to see an ophthalmologist for ocular dis- Once thought to be rare, sarcoidosis affects people ease, a cardiologist for cardiac disease, a neurologist for neu- throughout the world. It can affect people of any age, race, rological disease, a nephrologist for renal disease, and so or gender; however, the prevalence is highest among adults forth. between the ages of 20 and 40 and in African Americans and people of European – particularly Scandinavian – descent. Pharmacologic Treatment Symptoms and severity can vary by race and gender, with While a significant percentage of sarcoidosis patients never African Americans being more severely affected than need therapy, there are several groups which require treat- Caucasians. Extrapulmonary sarcoidosis is common in cer- ment. In this monograph, we will discuss several of the com- tain populations, for example: chronic uveitis in African monly used drugs for sarcoidosis and their potential toxici- Americans, painful skin lesions in Northern Europeans and ties, and will provide algorithms for use of these drugs to cardiac and ocular involvement in Japanese.