Diagnosis of Laryngeal Pemphigus Vulgaris Can Be Facilitated Using Advanced Endoscopic Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Use of Biologic Agents in the Treatment of Oral Lesions Due to Pemphigus and Behçet's Disease: a Systematic Review
Davis GE, Sarandev G, Vaughan AT, Al-Eryani K, Enciso R. The Use of Biologic Agents in the Treatment of Oral Lesions due to Pemphigus and Behçet’s Disease: A Systematic Review. J Anesthesiol & Pain Therapy. 2020;1(1):14-23 Systematic Review Open Access The Use of Biologic Agents in the Treatment of Oral Lesions due to Pemphigus and Behçet’s Disease: A Systematic Review Gerald E. Davis II1,2, George Sarandev1, Alexander T. Vaughan1, Kamal Al-Eryani3, Reyes Enciso4* 1Advanced graduate, Master of Science Program in Orofacial Pain and Oral Medicine, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA 2Assistant Dean of Academic Affairs, Assistant Professor, Restorative Dentistry, Meharry Medical College, School of Dentistry, Nashville, Tennessee, USA 3Assistant Professor of Clinical Dentistry, Division of Periodontology, Dental Hygiene & Diagnostic Sciences, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA 4Associate Professor (Instructional), Division of Dental Public Health and Pediatric Dentistry, Herman Ostrow School of Dentistry of USC, Los Angeles, California, USA Article Info Abstract Article Notes Background: Current treatments for pemphigus and Behçet’s disease, such Received: : March 11, 2019 as corticosteroids, have long-term serious adverse effects. Accepted: : April 29, 2020 Objective: The objective of this systematic review was to evaluate the *Correspondence: efficacy of biologic agents (biopharmaceuticals manufactured via a biological *Dr. Reyes Enciso, Associate Professor (Instructional), Division source) on the treatment of intraoral lesions associated with pemphigus and of Dental Public Health and Pediatric Dentistry, Herman Ostrow Behçet’s disease compared to glucocorticoids or placebo. School of Dentistry of USC, Los Angeles, California, USA; Email: [email protected]. -

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Zeroing in on the Cause of Your Patient's Facial Pain
Feras Ghazal, DDS; Mohammed Ahmad, Zeroing in on the cause MD; Hussein Elrawy, DDS; Tamer Said, MD Department of Oral Health of your patient's facial pain (Drs. Ghazal and Elrawy) and Department of Family Medicine/Geriatrics (Drs. Ahmad and Said), The overlapping characteristics of facial pain can make it MetroHealth Medical Center, Cleveland, Ohio difficult to pinpoint the cause. This article, with a handy at-a-glance table, can help. [email protected] The authors reported no potential conflict of interest relevant to this article. acial pain is a common complaint: Up to 22% of adults PracticE in the United States experience orofacial pain during recommendationS F any 6-month period.1 Yet this type of pain can be dif- › Advise patients who have a ficult to diagnose due to the many structures of the face and temporomandibular mouth, pain referral patterns, and insufficient diagnostic tools. disorder that in addition to Specifically, extraoral facial pain can be the result of tem- taking their medication as poromandibular disorders, neuropathic disorders, vascular prescribed, they should limit disorders, or atypical causes, whereas facial pain stemming activities that require moving their jaw, modify their diet, from inside the mouth can have a dental or nondental cause and minimize stress; they (FIGURE). Overlapping characteristics can make it difficult to may require physical therapy distinguish these disorders. To help you to better diagnose and and therapeutic exercises. C manage facial pain, we describe the most common causes and underlying pathological processes. › Consider prescribing a tricyclic antidepressant for patients with persistent idiopathic facial pain. C Extraoral facial pain Extraoral pain refers to the pain that occurs on the face out- 2-15 Strength of recommendation (SoR) side of the oral cavity. -

Study of Oropharyngeal Ulcers with Their Commonest Anatomical Sites of Presentation Correlated with Histopathological Diagnosis Among the North Bengal Population
Panacea Journal of Medical Sciences 2020;10(3):258–263 Content available at: https://www.ipinnovative.com/open-access-journals Panacea Journal of Medical Sciences Journal homepage: www.ipinnovative.com Original Research Article Study of oropharyngeal ulcers with their commonest anatomical sites of presentation correlated with histopathological diagnosis among the north Bengal population Tanwi Ghosal (Sen)1, Pallab Kr Saha2,*, Sauris Sen3 1Dept. of Anatomy, North Bengal Medical College, Sushrutanagar, West Bengal, India 2Dept. of Anatomy, NRS Medical College, Kolkata, West Bengal, India 3Dept. of ENT, Jalpaiguri District Hospital, Jalpaiguri, West Bengal, India ARTICLEINFO ABSTRACT Article history: Introduction: Oropharyngeal ulcers are very common in North East part of India due to bad oral habits like Received 31-07-2020 chewing of tobacco etc. A study was performed at North Bengal Medical College and Hospital among the Accepted 06-10-2020 patients attending otorhinolaryngology outdoor to explore the relationship of different types and proportion Available online 29-12-2020 of oropharyngeal ulcers with their commonest anatomical sites and to elicit the relationship with bad oral habits. Aims: • To elicit the different types and proportions of oropharyngeal ulcers. • To explore the relationship Keywords: of different histopathological types with anatomical sites. • To identify the relationship with addiction. Ulcer Material and Methods: This is a Cross-sectional Observational Hospital based study, conducted in the Oral Cavity Otorhinolaryngology outdoor of North Bengal Medical College and Hospital twice a week. 102 patients Oropharynx were selected as study population after maintaining inclusion and exclusion criteria. Anatomical sites Results: The study shows that the most common type of ulcer is aphthous (25.49%) and the least common types are erythroplakia and autoimmune ulcers (1.96%). -

Oral Manifestations of Systemic Disease Their Clinical Practice
ARTICLE Oral manifestations of systemic disease ©corbac40/iStock/Getty Plus Images S. R. Porter,1 V. Mercadente2 and S. Fedele3 provide a succinct review of oral mucosal and salivary gland disorders that may arise as a consequence of systemic disease. While the majority of disorders of the mouth are centred upon the focus of therapy; and/or 3) the dominant cause of a lessening of the direct action of plaque, the oral tissues can be subject to change affected person’s quality of life. The oral features that an oral healthcare or damage as a consequence of disease that predominantly affects provider may witness will often be dependent upon the nature of other body systems. Such oral manifestations of systemic disease their clinical practice. For example, specialists of paediatric dentistry can be highly variable in both frequency and presentation. As and orthodontics are likely to encounter the oral features of patients lifespan increases and medical care becomes ever more complex with congenital disease while those specialties allied to disease of and effective it is likely that the numbers of individuals with adulthood may see manifestations of infectious, immunologically- oral manifestations of systemic disease will continue to rise. mediated or malignant disease. The present article aims to provide This article provides a succinct review of oral manifestations a succinct review of the oral manifestations of systemic disease of of systemic disease. It focuses upon oral mucosal and salivary patients likely to attend oral medicine services. The review will focus gland disorders that may arise as a consequence of systemic upon disorders affecting the oral mucosa and salivary glands – as disease. -

Paraneoplastic Pemphigus with Clinical Features of Lichen Planus Associated with Low-Grade B Cell Lymphoma
Report Paraneoplastic pemphigus with clinical features of lichen planus associated with low-grade B cell lymphoma Sónia Coelho, MD, José Pedro Reis, MD, Oscar Tellechea, MD, PhD, Américo Figueiredo, MD, PhD, and Martin Black, MD, PhD From the Department of Dermatology, Abstract University Hospital, Coimbra, Portugal, St Background Neoplasia-induced lichen planus is described as a cell-mediated reaction to John’s Institute of Dermatology, St Thomas’ unknown epithelial antigens. Paraneoplastic pemphigus (PNP), characterized by the presence Hospital, London, UK of a specific array of autoantibodies, probably represents a different form of presentation of the Correspondence same autoimmune syndrome where the mucocutaneous expression depends on the dominant Sónia Coelho pathologic mechanism. Clínica de Dermatologia, Hospital da Methods The authors report a case of PNP with predominant lichen planus-like lesions and Universidade review the relevant literature. We observed a 74-year-old female with vesico-bullous, erosive, P.3000–075 Coimbra target-shaped and flat papular lichenoid lesions on the lower legs, palms and soles, evolving for Portugal E-mail: [email protected] 3 weeks. Histopathology revealed a lichenoid dermatitis. Direct immunofluorescence showed C3 deposition around keratinocytes and epidermal IgG intranuclear deposition. Indirect immunofluorescence revealed circulating IgG with intercellular staining on rat bladder substrate. Immunoblotting demonstrated bands of 130, 190, 210 and 250 kDa antigens. A pararenal B cell lymphoma was found. Results Oral corticotherapy with 40 mg prednisolone daily was initiated with a good cutaneous response. Four months later, cyclophosphamide (50 mg/day) was introduced because of a discrete enlargement of the pararenal mass. The patient died on the seventh month of follow up as a result of respiratory insufficiency. -

Cardiovascular Drugs-Induced Oral Toxicities: a Murky Area to Be Revisited and Illuminated
Pharmacological Research 102 (2015) 81–89 Contents lists available at ScienceDirect Pharmacological Research j ournal homepage: www.elsevier.com/locate/yphrs Review Cardiovascular drugs-induced oral toxicities: A murky area to be revisited and illuminated a, b b Pitchai Balakumar ∗, Muthu Kavitha , Suresh Nanditha a Pharmacology Unit, Faculty of Pharmacy, AIMST University, Semeling, 08100 Bedong, Malaysia b Faculty of Dentistry, AIMST University, 08100 Bedong, Malaysia a r t i c l e i n f o a b s t r a c t Article history: Oral health is an imperative part of overall human health. Oral disorders are often unreported, but are Received 20 July 2015 highly troublesome to human health in a long-standing situation. A strong association exists between Received in revised form 22 August 2015 cardiovascular drugs and oral adverse effects. Indeed, several cardiovascular drugs employed clinically Accepted 8 September 2015 have been reported to cause oral adverse effects such as xerostomia, oral lichen planus, angioedema, Available online 25 September 2015 aphthae, dysgeusia, gingival enlargement, scalded mouth syndrome, cheilitis, glossitis and so forth. Oral complications might in turn worsen the cardiovascular disease condition as some reports suggest an Keywords: adverse correlation between periodontal oral disease pathogenesis and cardiovascular disease. These are Cardiovascular drugs certainly important to be understood for a better use of cardiovascular medicines and control of associated Oral adverse effects oral adverse effects. This review sheds lights on the oral adverse effects pertaining to the clinical use of Dry mouth Angioedema cardiovascular drugs. Above and beyond, an adverse correlation between oral disease and cardiovascular Dysgeusia disease has been discussed. -

Pemphigus. S2 Guideline for Diagnosis and Treatment
DOI: 10.1111/jdv.12772 JEADV GUIDELINES Pemphigus. S2 Guideline for diagnosis and treatment – guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV) M. Hertl,1,* H. Jedlickova,2 S. Karpati,3 B. Marinovic,4 S. Uzun,5 S. Yayli,6 D. Mimouni,7 L. Borradori,8 C. Feliciani,9 D. Ioannides,10 P. Joly,11 C. Kowalewski,12 G. Zambruno,13 D. Zillikens,14 M.F. Jonkman15 1Department of Dermatology, Philipps-University Marburg, Marburg, Germany 2Department of Dermatology, Masaryk University, Brno, Czech Republic 3Department of Dermatology, Semmelweis University Budapest, Budapest, Hungary 4Department of Dermatology, School of Medicine University of Zagreb, Zagreb, Croatia 5Department of Dermatology, Akdeniz University, Antalya, Turkey 6Department of Dermatology, Karadeniz Technical University, Trabzon, Turkey 7Department of Dermatology, Tel-Aviv University, Tel-Aviv, Israel 8Department of Dermatology, University of Bern, Inselspital, Switzerland 9Department of Dermatology, University of Parma, Parma, Italy 10Department of Dermatology, Aristotle University of Thessaloniki, Thessaloniki, Greece 11Department of Dermatology, Rouen University Hospital, Rouen, France 12Department of Dermatology, Medical University of Warsaw, Warsaw, Poland 13Department of Dermatology, L’Istituto Dermopatico dell’Immacolata, Rome, Italy 14Department of Dermatology, University of Lubeck,€ Lubeck,€ Germany 15Department of Dermatology, University of Groningen, Groningen, The Netherlands *Correspondence: M. Hertl. E-mail: [email protected] Abstract Background Pemphigus encompasses a group of life-threatening autoimmune bullous diseases characterized by blis- ters and erosions of the mucous membranes and skin. Before the era of immunosuppressive treatment, the prognosis of pemphigus was almost fatal. Due to its rarity, only few prospective controlled therapeutic trials are available. -

Oral Manifestations of Pemphigus Vulgaris
Journal of Clinical & Experimental Dermatology Research - Open Access Research Article OPEN ACCESS Freely available online doi:10.4172/2155-9554.1000112 Oral Manifestations of Pemphigus Vulgaris: Clinical Presentation, Differential Diagnosis and Management Antonio Bascones-Martinez1*, Marta Munoz-Corcuera2, Cristina Bascones-Ilundain1 and German Esparza-Gómez1 1DDS, PhD, Medicine and Bucofacial Surgery Department, Dental School, Complutense University of Madrid, Spain 2DDS, PhD Student, Medicine and Bucofacial Surgery Department, Dental School, Complutense University of Madrid, Spain Abstract Pemphigus vulgaris is a chronic autoimmune mucocutaneous disease characterized by the formation of intraepithelial blisters. It results from an autoimmune process in which antibodies are produced against desmoglein 1 and desmoglein 3, normal components of the cell membrane of keratinocytes. The first manifestations of pemphigus vulgaris appear in the oral mucosa in the majority of patients, followed at a later date by cutaneous lesions. The diagnosis is based on clinical findings and laboratory analyses, and it is usually treated by the combined administration of corticosteroids and immunosuppressants. Detection of the oral lesions can result in an earlier diagnosis. We review the oral manifestations of pemphigus vulgaris as well as the differential diagnosis, treatment, and prognosis of oral lesions in this uncommon disease. Keywords: Pemphigus; Oral mucosa; Autoimmune bullous disease and have a molecular weight of 130 and 160 KDa, respectively [1,7,9,13]. The binding of antibodies to desmoglein at mucosal or Introduction cutaneous level gives rise to the loss of cell adhesion, with separation of epithelial layers (acantholysis) and the consequent appearance of Pemphigus vulgaris (PV) is the most frequently observed blisters on skin or mucosae [1,3]. -

Hairy Leukoplakia James E
Marquette University e-Publications@Marquette School of Dentistry Faculty Research and Dentistry, School of Publications 5-5-2017 Hairy Leukoplakia James E. Cade Meharry Medical College School of Dentistry Richard P. Vinson Paul L Foster School of Medicine Jeff urB gess University of Washington School of Dental Medicine Sanjiv S. Agarwala Temple University Shool of Medicine Denis P. Lynch Marquette University, [email protected] See next page for additional authors Published version. Medscape Drugs & Diseases (May 5, 2017). Publisher link. © 2017 by WebMD LLC. Used with permission. Authors James E. Cade, Richard P. Vinson, Jeff urB gess, Sanjiv S. Agarwala, Denis P. Lynch, and Gary L. Stafford This blog post/website is available at e-Publications@Marquette: https://epublications.marquette.edu/dentistry_fac/252 Overview Background Oral hairy leukoplakia (OHL) is a disease of the mucosa first described in 1984. This pathology is associated with Epstein-Barr virus (EBV) and occurs mostly in people with HIV infection, both immunocompromised and immunocompetent, and can affect patients who are HIV negative.{ref1}{ref2} The first case in an HIV-negative patient was reported in 1999 in a 56-year-old patient with acute lymphocytic leukemia. Later, many cases were reported in heart, kidney, and bone marrow transplant recipients and patients with hematological malignancies.{ref3}{ref4} Pathophysiology The Epstein-Barr virus (EBV), a ubiquitous herpesvirus estimated to infect 90% of the world's population, is linked to a growing number of diseases, especially in immunocompromised hosts. Like all herpesviruses, EBV establishes a life-long, persistent infection of its host. The pathogenesis of hairy leukoplakia is clearly complex, potentially requiring a convergence of factors including EBV co-infection, productive EBV replication, EBV genetic evolution, expression of specific EBV "latent" genes, and immune escape. -
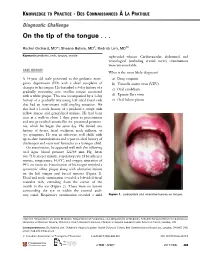
On the Tip of the Tongue
KNOWLEDGE TO PRACTICE DES CONNAISSANCES ÀLA PRATIQUE Diagnostic Challenge On the tip of the tongue . Rachel Orchard, MD*; Sheena Belisle, MD†; Rodrick Lim, MD†‡ Keywords: pediatric, rash, tongue, vesicle right-sided wheeze. Cardiovascular, abdominal, and neurological (including cranial nerve) examinations were unremarkable. CASE HISTORY What is the most likely diagnosis? A 14-year-old male presented to the pediatric emer- a) Drug eruption gency department (ED) with a chief complaint of b) Varicella zoster virus (VZV) changes to his tongue. He described a 3-day history of a c) Oral candidiasis gradually worsening sore, swollen tongue associated with a white plaque. This was accompanied by a 3-day d) Epstein-Barr virus history of a gradually worsening left-sided facial rash e) Oral lichen planus that had an intermittent mild tingling sensation. He also had a 1-week history of a productive cough with yellow mucus and generalized malaise. He had been seen at a walk-in clinic 2 days prior to presentation and was prescribed amoxicillin for presumed pneumo- nia, which he began the same day. He denied any history of fevers, facial weakness, neck stiffness, or eye symptoms. He was an otherwise well child, with up-to-date immunizations and a past medical history of chickenpox and recurrent furuncles as a younger child. On examination, he appeared well with the following vital signs: blood pressure 122/64 mm Hg, heart rate 73 beats per minute, respiratory rate 18 breaths per minute, temperature 36.8°C, and oxygen saturation of 99% on room air. Examination of his tongue revealed a symmetric white plaque along with ulcerative lesions on the left tongue and buccal mucosa (Figure 1). -

Oral Leukoplakia
Division of Oral Medicine and Dentistry Oral Leukoplakia What is oral leukoplakia? What causes oral leukoplakia? Oral leukoplakia (leuko=white, plakia=patch) is a white patch in Alcohol and tobacco use, both known risk factors for the mouth that cannot be rubbed of and cannot be diagnosed oral cancer, are similarly well-established risk factors for as any other condition. Lichen planus, yeast infections development of oral leukoplakia. Other risk factors include a (“thrush”), chronic cheek and tongue chewing injuries, and weakened immune system, long-term treatment with immune hairy/coated tongue are some of the specifc conditions suppressing medications, a personal or family history of cancer, that appear white in the mouth and are therefore NOT oral and, in some cultures, the chewing of areca nut and betel leaf. In leukoplakia. When all such known conditions have been ruled many patients with oral leukoplakia, however, there are no risk out, a patient is diagnosed with oral leukoplakia. While the factors and we don’t know why it develops. long-term history of these lesions is impossible to predict, it How do we know it is oral leukoplakia? is known that true leukoplakias are considered “potentially malignant,” meaning that they have the potential, over time, to If your doctor suspects that a white lesion in your mouth is due develop into oral cancer. to irritation, the source of the irritation will be removed and you will be asked to return in a few weeks for re-evaluation. If the Oral leukoplakia occurs in 1-2% of the population and is most white area is still present at the next visit, a biopsy will likely be common in patients over age 40.