5-HT Receptors and the Development of New Antidepressants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Striking the Right Balance Between Gi and Gq Signalling a New Study Explains Why Some Inhibitors of the Serotonin
RESEARCH HIGHLIGHTS IN BRIEF PERCEPTION Excitability modulates synaesthesia Synaesthetes who experience colours when perceiving or representing numbers exhibit structural and functional differences in cortical areas that are involved in number and colour processing compared to non-synaesthetes. Using transcranial magnetic stimulation or transcranial direct current stimulation, the authors showed that in humans, grapheme-colour synaesthesia is characterized by enhanced cortical excitability in the primary visual cortex and can be augmented or attenuated with cathodal or anodal stimulation, respectively. ORIGINAL RESEARCH PAPER Terhune, D. B. et al. Enhanced cortical excitability in grapheme-color synesthesia and its modulation. Curr. Biol. 21, 2006–2009 (2011) NEUROPHARMACOLOGY Striking the right balance between Gi and Gq signalling A new study explains why some inhibitors of the serotonin Gq protein-coupled receptor 2AR (such as clozapine), but not others (such as ritanserin), have antipsychotic actions. The authors showed that 2AR can form a heteromeric complex with metabotropic glutamate receptor 2 (mGluR2) — which is a Gi protein-coupled receptor — and that this interaction can enhance glutamate-induced Gi signalling and reduce serotonin-induced Gq signalling. The intracellular Gi and Gq signalling balance is predictive of the anti- or pro-psychotic activity of drugs targeting 2AR and mGluR2, providing a potential new metric to improve current antipsychotic therapies. ORIGINAL RESEARCH PAPER Fribourg, M. et al. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147, 1011–1023 (2011) BEHAVIOURAL NEUROSCIENCE Curbing sweet cravings In this study, the authors examined the reward value of sweeteners in mice by assessing the animals’ preferences for sweeteners compared to lick-induced optogenetic activation of midbrain dopaminergic neurons. -

Serotonin's Role in Alcohol's Effects on the Brain
NEUROTRANSMITTER REVIEW IMPERATO, A., AND DI CHIARA, G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. Journal SEROTONIN’S ROLE IN ALCOHOL’S of Pharmacology and Experimental Therapeutics 239:219–228, 1986. EFFECTS ON THE BRAIN KITAI, S.T., AND SURMEIER, D.J. Cholinergic and dopaminergic modula- tion of potassium conductances in neostriatal neurons. Advances in David M. Lovinger, Ph.D. Neurology 60:40–52, 1993. LE MOINE, C.; NORMAND, E.; GUITTENY, A.F.; FOUQUE, B.; TEOULE, R.; AND BLOCH, B. Dopamine receptor gene expression by enkephalin Serotonin is an important brain chemical that acts as a neurons in rat forebrain. Proceedings of the National Academy of Sciences neurotransmitter to communicate information among USA 87:230–234, 1990. nerve cells. Serotonin’s actions have been linked to al- LE MOINE, C.; NORMAND, E.; AND BLOCH, B. Phenotypical character- cohol’s effects on the brain and to alcohol abuse. ization of the rat striatal neurons expressing the D1 dopamine receptor Alcoholics and experimental animals that consume gene. Proceedings of the National Academy of Sciences USA 88: large quantities of alcohol show evidence of differences 4205–4209, 1991. in brain serotonin levels compared with nonalcoholics. LYNESS, W.H., AND SMITH F.L. Influence of dopaminergic and sero- Both short- and long-term alcohol exposure also affect tonergic neurons on intravenous ethanol self-administration in the rat. the serotonin receptors that convert the chemical sig- Pharmacology and Biochemistry of Behavior 42:187–192, 1992. nal produced by serotonin into functional changes in the signal-receiving cell. -

Molecular Signatures of G-Protein-Coupled Receptors A
REVIEW doi:10.1038/nature11896 Molecular signatures of G-protein-coupled receptors A. J. Venkatakrishnan1, Xavier Deupi2, Guillaume Lebon1,3,4,5, Christopher G. Tate1, Gebhard F. Schertler2,6 & M. Madan Babu1 G-protein-coupled receptors (GPCRs) are physiologically important membrane proteins that sense signalling molecules such as hormones and neurotransmitters, and are the targets of several prescribed drugs. Recent exciting developments are providing unprecedented insights into the structure and function of several medically important GPCRs. Here, through a systematic analysis of high-resolution GPCR structures, we uncover a conserved network of non-covalent contacts that defines the GPCR fold. Furthermore, our comparative analysis reveals characteristic features of ligand binding and conformational changes during receptor activation. A holistic understanding that integrates molecular and systems biology of GPCRs holds promise for new therapeutics and personalized medicine. ignal transduction is a fundamental biological process that is comprehensively, and in the process expand the current frontiers of required to maintain cellular homeostasis and to ensure coordi- GPCR biology. S nated cellular activity in all organisms. Membrane proteins at the In this analysis, we objectively compare known structures and reveal cell surface serve as the communication interface between the cell’s key similarities and differences among diverse GPCRs. We identify a external and internal environments. One of the largest and most diverse consensus structural scaffold of GPCRs that is constituted by a network membrane protein families is the GPCRs, which are encoded by more of non-covalent contacts between residues on the transmembrane (TM) than 800 genes in the human genome1. GPCRs function by detecting a helices. -

Emerging Evidence for a Central Epinephrine-Innervated A1- Adrenergic System That Regulates Behavioral Activation and Is Impaired in Depression
Neuropsychopharmacology (2003) 28, 1387–1399 & 2003 Nature Publishing Group All rights reserved 0893-133X/03 $25.00 www.neuropsychopharmacology.org Perspective Emerging Evidence for a Central Epinephrine-Innervated a1- Adrenergic System that Regulates Behavioral Activation and is Impaired in Depression ,1 1 1 1 1 Eric A Stone* , Yan Lin , Helen Rosengarten , H Kenneth Kramer and David Quartermain 1Departments of Psychiatry and Neurology, New York University School of Medicine, New York, NY, USA Currently, most basic and clinical research on depression is focused on either central serotonergic, noradrenergic, or dopaminergic neurotransmission as affected by various etiological and predisposing factors. Recent evidence suggests that there is another system that consists of a subset of brain a1B-adrenoceptors innervated primarily by brain epinephrine (EPI) that potentially modulates the above three monoamine systems in parallel and plays a critical role in depression. The present review covers the evidence for this system and includes findings that brain a -adrenoceptors are instrumental in behavioral activation, are located near the major monoamine cell groups 1 or target areas, receive EPI as their neurotransmitter, are impaired or inhibited in depressed patients or after stress in animal models, and a are restored by a number of antidepressants. This ‘EPI- 1 system’ may therefore represent a new target system for this disorder. Neuropsychopharmacology (2003) 28, 1387–1399, advance online publication, 18 June 2003; doi:10.1038/sj.npp.1300222 Keywords: a1-adrenoceptors; epinephrine; motor activity; depression; inactivity INTRODUCTION monoaminergic systems. This new system appears to be impaired during stress and depression and thus may Depressive illness is currently believed to result from represent a new target for this disorder. -
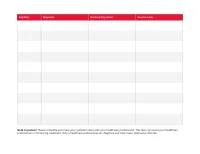
Print Your Symptom Diary
MEDICATION GUIDE FETZIMA® (fet-ZEE-muh) (levomilnacipran) extended-release capsules, for oral use What is the most important information I should know about FETZIMA? FETZIMA may cause serious side effects, including: • Increased risk of suicidal thoughts or actions in some children, adolescents, and young adults. FETZIMA and other antidepressant medicines may increase suicidal thoughts or actions in some children and young adults, especially within the first few months of treatment or when the dose is changed. FETZIMA is not for use in children. o Depression or other serious mental illnesses are the most important causes of suicidal thoughts or actions. Some people may have a higher risk of having suicidal thoughts or actions. These include people who have (or have a family history of) depression, bipolar illness (also called manic-depressive illness) or have a history of suicidal thoughts or actions. How can I watch for and try to prevent suicidal thoughts and actions? o Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings, or if you develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed. o Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings. o Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms. Call your healthcare provider or -

Antidepressant Discontinuation Syndrome CHRISTOPHER H
Antidepressant Discontinuation Syndrome CHRISTOPHER H. WARNER, MAJ, MC, USA, Winn Army Community Hospital, Fort Stewart, Georgia WILLIAM BOBO, LCDR, MC, USN, Uniformed Services University of the Health Sciences, Bethesda, Maryland CAROLYNN WARNER, MAJ, MC, USA, Winn Army Community Hospital, Fort Stewart, Georgia SARA REID, CPT, USAF, MC, Bolling Air Force Base, Washington, D.C. JAMES RACHAL, MAJ, USAF, MC, Ehrling Berquist Air Force Hospital, Offutt Air Force Base, Omaha, Nebraska Antidepressant discontinuation syndrome occurs in approximately 20 percent of patients after abrupt discontinuation of an antidepressant medication that was taken for at least six weeks. Typical symptoms of antidepressant discontinuation syndrome include flu-like symptoms, insomnia, nausea, imbalance, sensory disturbances, and hyperarousal. These symptoms usu- ally are mild, last one to two weeks, and are rapidly extinguished with reinstitution of antide- pressant medication. Antidepressant discontinuation syndrome is more likely with a longer duration of treatment and a shorter half-life of the treatment drug. A high index of suspicion should be maintained for the emergence of discontinuation symptoms, which should prompt close questioning regarding accidental or purposeful self-discontinuation of medication. Before antidepressants are prescribed, patient education should include warnings about the potential problems associated with abrupt discontinuation. Education about this common and likely underrecognized clinical phenomenon will help prevent future episodes -

A Novel Atypical Antidepressant That May Provide New Insights Into the Biomolecular Basis of Depression
Recent Patents on CNS Drug Discovery, 2006, 1, 29-41 29 Tianeptine: A Novel Atypical Antidepressant that May Provide New Insights into the Biomolecular Basis of Depression Christiaan B. Brink*, Brian H. Harvey and Linda Brand Division of Pharmacology, North-West University (PUK), Potchefstroom, 2520, South Africa Received: July 29, 2005; Accepted: September 05, 2005; Revised: September 12, 2005 Abstract: Tianeptine, an atypical antidepressant patented and developed by Servier, enhances the synaptic reuptake of serotonin, without affecting norepinephrine and dopamine uptake, while it lacks affinity for neurotransmitter receptors. This mechanism for an antidepressant is apparently paradoxical, since the currently employed antidepressants enhance serotonin by inhibiting its breakdown or by inhibiting monoaminergic reuptake. Although tianeptine has been shown to reduce central 5HT availability and to indirecty modulate central adrenergic and dopaminergic systems and to indirectly inhibit cholinergic hyperactivity, its antidepressant action is believed to be more directly related to central neuronal remodeling and restoration of neuronal plasticity. In reliable animal models of depression tianeptine has been shown to prevent neurodegeneration and decreases in hippocampal volume in response to chronic stress. These effects on neuroplasticity are suspected to involve the normalization of the hypothalamic-pituitary-adrenal axis and modulatory effects on excitatory amino acids and N-methyl-D-aspartate receptors. Together with a body of related studies, these data provide further support for the hypothesis that depression may involve dysregulation of pathways controlling cellular resilience and that treatment should be directed towards the reversal thereof. Importantly, tianeptine is not anxiogenic and has also been shown to be effective in treatment-resistant depression, which may lead the way to a major breakthrough in the treatment of depression. -

Understanding the Role of GPCR Heteroreceptor Complexes in Modulating the Brain Networks in Health and Disease
REVIEW published: 21 February 2017 doi: 10.3389/fncel.2017.00037 Understanding the Role of GPCR Heteroreceptor Complexes in Modulating the Brain Networks in Health and Disease Dasiel O. Borroto-Escuela 1,2,3, Jens Carlsson 4, Patricia Ambrogini 2, Manuel Narváez 5, Karolina Wydra 6, Alexander O. Tarakanov 7, Xiang Li 1, Carmelo Millón 5, Luca Ferraro 8, Riccardo Cuppini 2, Sergio Tanganelli 9, Fang Liu 10, Malgorzata Filip 6, Zaida Diaz-Cabiale 5 and Kjell Fuxe 1* 1Department of Neuroscience, Karolinska Institutet, Stockholm, Sweden, 2Department of Biomolecular Science, Section of Physiology, University of Urbino, Urbino, Italy, 3Observatorio Cubano de Neurociencias, Grupo Bohío-Estudio, Yaguajay, Cuba, 4Department of Cell and Molecular Biology, Uppsala Biomedical Centre (BMC), Uppsala University, Uppsala, Sweden, 5Facultad de Medicina, Instituto de Investigación Biomédica de Málaga, Universidad de Málaga, Málaga, Spain, 6Laboratory of Drug Addiction Pharmacology, Department of Pharmacology, Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland, 7St. Petersburg Institute for Informatics and Automation, Russian Academy of Sciences, Saint Petersburg, Russia, 8Department of Life Sciences and Biotechnology, University of Ferrara, Ferrara, Italy, 9Department of Medical Sciences, University of Ferrara, Ferrara, Italy, 10Campbell Research Institute, Centre for Addiction and Mental Health, University of Toronto, Toronto, ON, Canada The introduction of allosteric receptor–receptor interactions in G protein-coupled receptor (GPCR) heteroreceptor complexes of the central nervous system (CNS) gave a new dimension to brain integration and neuropsychopharmacology. The molecular basis Edited by: Hansen Wang, of learning and memory was proposed to be based on the reorganization of the homo- University of Toronto, Canada and heteroreceptor complexes in the postjunctional membrane of synapses. -

Cysteinyl Leukotriene Receptor 1/2 Antagonists Nonselectively Modulate Organic Anion Transport by Multidrug Resistance Proteins (MRP1-4) S
Supplemental material to this article can be found at: http://dmd.aspetjournals.org/content/suppl/2016/04/11/dmd.116.069468.DC1 1521-009X/44/6/857–866$25.00 http://dx.doi.org/10.1124/dmd.116.069468 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 44:857–866, June 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics Cysteinyl Leukotriene Receptor 1/2 Antagonists Nonselectively Modulate Organic Anion Transport by Multidrug Resistance Proteins (MRP1-4) s Mark A. Csandl, Gwenaëlle Conseil, and Susan P. C. Cole Departments of Biomedical and Molecular Sciences (M.A.C., S.P.C.C.), and Pathology and Molecular Medicine (G.C., S.P.C.C.), Division of Cancer Biology and Genetics, Queen’s University Cancer Research Institute, Kingston, ON, Canada Received January 13, 2016; accepted April 7, 2016 ABSTRACT Active efflux of both drugs and organic anion metabolites is class of antagonists showed any MRP selectivity. For E217bG Downloaded from mediated by the multidrug resistance proteins (MRPs). MRP1 uptake, LTM IC50s ranged from 1.2 to 26.9 mMandweremost (ABCC1), MRP2 (ABCC2), MRP3 (ABCC3), and MRP4 (ABCC4) have comparable for MRP1 and MRP4. The LTM rank order inhibitory partially overlapping substrate specificities and all transport 17b- potencies for E217bGversusLTC4 uptake by MRP1, and E217bG estradiol 17-(b-D-glucuronide) (E217bG). The cysteinyl leukotriene versus PGE2 uptake by MRP4, were also similar. Three of four receptor 1 (CysLT1R) antagonist MK-571 inhibits all four MRP CysLT1R-selective LTMs also stimulated MRP2 (but not MRP3) homologs, but little is known about the modulatory effects of newer transport and thus exerted a concentration-dependent biphasic leukotriene modifiers (LTMs). -

The 5-HT6 Receptor Antagonist SB-271046 Selectively Enhances Excitatory Neurotransmission in the Rat Frontal Cortex and Hippocampus Lee A
The 5-HT6 Receptor Antagonist SB-271046 Selectively Enhances Excitatory Neurotransmission in the Rat Frontal Cortex and Hippocampus Lee A. Dawson, Ph.D., Huy Q. Nguyen, B.S., and Ping Li, B.S. Preclinical evidence has suggested a possible role for the 5-HT6 increases in extracellular glutamate levels in both frontal receptor in the treatment of cognitive dysfunction. However, cortex and dorsal hippocampus, respectively. These effects were currently there is little neurochemical evidence suggesting the completely attenuated by infusion of tetrodotoxin but mechanism(s) which may be involved. Using the selective unaffected by the muscarinic antagonist, atropine. Here we 5-HT6 antagonist SB-271046 and in vivo microdialysis, we demonstrate for the first time the selective enhancement of have evaluated the effects of this compound on the modulation excitatory neurotransmission by SB-271046 in those brain of basal neurotransmitter release within multiple brain regions regions implicated in cognitive and memory function, and of the freely moving rat. SB-271046 produced no change in provide mechanistic evidence in support of a possible basal levels of dopamine (DA), norepinephrine (NE) or 5-HT therapeutic role for 5-HT6 receptor antagonists in the in the striatum, frontal cortex, dorsal hippocampus or nucleus treatment of cognitive and memory dysfunction. accumbens. Similarly, this compound had no effect on [Neuropsychopharmacology 25:662–668, 2001] excitatory neurotransmission in the striatum or nucleus © 2001 American College of Neuropsychopharmacology. accumbens. Conversely, SB-271046 produced 3- and 2-fold Published by Elsevier Science Inc. KEY WORDS: 5-HT6 receptor; SB-271046; Microdialysis; sma et al. 1993; Ruat et al. -

The Serotonergic System in Migraine Andrea Rigamonti Domenico D’Amico Licia Grazzi Susanna Usai Gennaro Bussone
J Headache Pain (2001) 2:S43–S46 © Springer-Verlag 2001 MIGRAINE AND PATHOPHYSIOLOGY Massimo Leone The serotonergic system in migraine Andrea Rigamonti Domenico D’Amico Licia Grazzi Susanna Usai Gennaro Bussone Abstract Serotonin (5-HT) and induce migraine attacks. Moreover serotonin receptors play an impor- different pharmacological preventive tant role in migraine pathophysiolo- therapies (pizotifen, cyproheptadine gy. Changes in platelet 5-HT content and methysergide) are antagonist of are not casually related, but they the same receptor class. On the other may reflect similar changes at a neu- side the activation of 5-HT1B-1D ronal level. Seven different classes receptors (triptans and ergotamines) of serotoninergic receptors are induce a vasocostriction, a block of known, nevertheless only 5-HT2B-2C neurogenic inflammation and pain M. Leone • A. Rigamonti • D. D’Amico and 5HT1B-1D are related to migraine transmission. L. Grazzi • S. Usai • G. Bussone (౧) syndrome. Pharmacological evi- C. Besta National Neurological Institute, Via Celoria 11, I-20133 Milan, Italy dences suggest that migraine is due Key words Serotonin • Migraine • e-mail: [email protected] to an hypersensitivity of 5-HT2B-2C Triptans • m-Chlorophenylpiperazine • Tel.: +39-02-2394264 receptors. m-Chlorophenylpiperazine Pathogenesis Fax: +39-02-70638067 (mCPP), a 5-HT2B-2C agonist, may The 5-HT receptor family is distinguished from all other 5- Introduction 1 HT receptors by the absence of introns in the genes; in addi- tion all are inhibitors of adenylate cyclase [1]. Serotonin (5-HT) and serotonin receptors play an important The 5-HT1A receptor has a high selective affinity for 8- role in migraine pathophysiology. -

Basal Ganglia & Cerebellum
1/2/2019 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the internet or on any personal websites. Your use of this resource is with the acknowledgment and acceptance of those restrictions. Basal Ganglia & Cerebellum – a quick overview MHD-Neuroanatomy – Neuroscience Block Gregory Gruener, MD, MBA, MHPE Vice Dean for Education, SSOM Professor, Department of Neurology LUHS a member of Trinity Health Outcomes you want to accomplish Basal ganglia review Define and identify the major divisions of the basal ganglia List the major basal ganglia functional loops and roles List the components of the basal ganglia functional “circuitry” and associated neurotransmitters Describe the direct and indirect motor pathways and relevance/role of the substantia nigra compacta 1 1/2/2019 Basal Ganglia Terminology Striatum Caudate nucleus Nucleus accumbens Putamen Globus pallidus (pallidum) internal segment (GPi) external segment (GPe) Subthalamic nucleus Substantia nigra compact part (SNc) reticular part (SNr) Basal ganglia “circuitry” • BG have no major outputs to LMNs – Influence LMNs via the cerebral cortex • Input to striatum from cortex is excitatory – Glutamate is the neurotransmitter • Principal output from BG is via GPi + SNr – Output to thalamus, GABA is the neurotransmitter • Thalamocortical projections are excitatory – Concerned with motor “intention” • Balance of excitatory & inhibitory inputs to striatum, determine whether thalamus is suppressed BG circuits are parallel loops • Motor loop – Concerned with learned movements • Cognitive loop – Concerned with motor “intention” • Limbic loop – Emotional aspects of movements • Oculomotor loop – Concerned with voluntary saccades (fast eye-movements) 2 1/2/2019 Basal ganglia “circuitry” Cortex Striatum Thalamus GPi + SNr Nolte.