Analyzing the Impact of Natural Gas Pipeline Corridors on Headwater Stream Productivity in Central Pennsylvania
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Susquehanna Riyer Drainage Basin
'M, General Hydrographic Water-Supply and Irrigation Paper No. 109 Series -j Investigations, 13 .N, Water Power, 9 DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY CHARLES D. WALCOTT, DIRECTOR HYDROGRAPHY OF THE SUSQUEHANNA RIYER DRAINAGE BASIN BY JOHN C. HOYT AND ROBERT H. ANDERSON WASHINGTON GOVERNMENT PRINTING OFFICE 1 9 0 5 CONTENTS. Page. Letter of transmittaL_.__.______.____.__..__.___._______.._.__..__..__... 7 Introduction......---..-.-..-.--.-.-----............_-........--._.----.- 9 Acknowledgments -..___.______.._.___.________________.____.___--_----.. 9 Description of drainage area......--..--..--.....-_....-....-....-....--.- 10 General features- -----_.____._.__..__._.___._..__-____.__-__---------- 10 Susquehanna River below West Branch ___...______-_--__.------_.--. 19 Susquehanna River above West Branch .............................. 21 West Branch ....................................................... 23 Navigation .--..........._-..........-....................-...---..-....- 24 Measurements of flow..................-.....-..-.---......-.-..---...... 25 Susquehanna River at Binghamton, N. Y_-..---...-.-...----.....-..- 25 Ghenango River at Binghamton, N. Y................................ 34 Susquehanna River at Wilkesbarre, Pa......_............-...----_--. 43 Susquehanna River at Danville, Pa..........._..................._... 56 West Branch at Williamsport, Pa .._.................--...--....- _ - - 67 West Branch at Allenwood, Pa.....-........-...-.._.---.---.-..-.-.. 84 Juniata River at Newport, Pa...-----......--....-...-....--..-..---.- -

2018 Pennsylvania Summary of Fishing Regulations and Laws PERMITS, MULTI-YEAR LICENSES, BUTTONS
2018PENNSYLVANIA FISHING SUMMARY Summary of Fishing Regulations and Laws 2018 Fishing License BUTTON WHAT’s NeW FOR 2018 l Addition to Panfish Enhancement Waters–page 15 l Changes to Misc. Regulations–page 16 l Changes to Stocked Trout Waters–pages 22-29 www.PaBestFishing.com Multi-Year Fishing Licenses–page 5 18 Southeastern Regular Opening Day 2 TROUT OPENERS Counties March 31 AND April 14 for Trout Statewide www.GoneFishingPa.com Use the following contacts for answers to your questions or better yet, go onlinePFBC to the LOCATION PFBC S/TABLE OF CONTENTS website (www.fishandboat.com) for a wealth of information about fishing and boating. THANK YOU FOR MORE INFORMATION: for the purchase STATE HEADQUARTERS CENTRE REGION OFFICE FISHING LICENSES: 1601 Elmerton Avenue 595 East Rolling Ridge Drive Phone: (877) 707-4085 of your fishing P.O. Box 67000 Bellefonte, PA 16823 Harrisburg, PA 17106-7000 Phone: (814) 359-5110 BOAT REGISTRATION/TITLING: license! Phone: (866) 262-8734 Phone: (717) 705-7800 Hours: 8:00 a.m. – 4:00 p.m. The mission of the Pennsylvania Hours: 8:00 a.m. – 4:00 p.m. Monday through Friday PUBLICATIONS: Fish and Boat Commission is to Monday through Friday BOATING SAFETY Phone: (717) 705-7835 protect, conserve, and enhance the PFBC WEBSITE: Commonwealth’s aquatic resources EDUCATION COURSES FOLLOW US: www.fishandboat.com Phone: (888) 723-4741 and provide fishing and boating www.fishandboat.com/socialmedia opportunities. REGION OFFICES: LAW ENFORCEMENT/EDUCATION Contents Contact Law Enforcement for information about regulations and fishing and boating opportunities. Contact Education for information about fishing and boating programs and boating safety education. -

Lycoming County
LYCOMING COUNTY START BRIDGE SD MILES PROGRAM IMPROVEMENT TYPE TITLE DESCRIPTION COST PERIOD COUNT COUNT IMPROVED Bridge rehabilitation on State Route 2014 over Lycoming Creek in the City of BASE Bridge Rehabilitation State Route 2014 over Lycoming Creek Williamsport 1 $ 2,100,000 1 0 0 Bridge replacement on PA 973 over the First Fork of Larry's Creek in Mifflin BASE Bridge Replacement PA 973 over the First Fork of Larry's Creek Township and epoxy overlay on PA 973 over Larry's Creek in Mifflin Township 1 $ 1,577,634 2 1 0 BASE Bridge Rehabilitation State Route 2039 over Mill Creek Bridge replacement on State Route 2039 over Mill Creek in Loyalsock Township 1 $ 398,640 1 1 0 Bridge rehabilitation on Township Road 434 over Mosquito Creek in Armstrong BASE Bridge Rehabilitation Township Road 434 over Mosquito Creek Township 3 $ 1,220,000 1 1 0 Bridge truss rehabilitation on State Route 2069 over Little Muncy Creek in BASE Bridge Rehabilitation State Route 2069 over Little Muncy Creek Moreland Township 1 $ 1,000,000 1 1 0 Bridge replacement on PA 87 over Tributary to Loyalsock Creek in Upper Fairfield BASE Bridge Replacement PA 87 over Tributary to Loyalsock Creek Township 3 $ 1,130,000 1 1 0 Bridge replacement on State Route 2001 (Elimsport Road) over Branch of Spring BASE Bridge Replacement State Route 2001 over Branch of Spring Creek #1 Creek in Washington Township 1 $ 1,270,000 1 1 0 BASE Bridge Replacement PA 414 over Upper Pine Bottom Run Bridge replacement on PA 414 over Upper Pine Bottom Run in Cummings Township 2 $ 1,620,000 1 1 -

Wild Trout Waters (Natural Reproduction) - September 2021
Pennsylvania Wild Trout Waters (Natural Reproduction) - September 2021 Length County of Mouth Water Trib To Wild Trout Limits Lower Limit Lat Lower Limit Lon (miles) Adams Birch Run Long Pine Run Reservoir Headwaters to Mouth 39.950279 -77.444443 3.82 Adams Hayes Run East Branch Antietam Creek Headwaters to Mouth 39.815808 -77.458243 2.18 Adams Hosack Run Conococheague Creek Headwaters to Mouth 39.914780 -77.467522 2.90 Adams Knob Run Birch Run Headwaters to Mouth 39.950970 -77.444183 1.82 Adams Latimore Creek Bermudian Creek Headwaters to Mouth 40.003613 -77.061386 7.00 Adams Little Marsh Creek Marsh Creek Headwaters dnst to T-315 39.842220 -77.372780 3.80 Adams Long Pine Run Conococheague Creek Headwaters to Long Pine Run Reservoir 39.942501 -77.455559 2.13 Adams Marsh Creek Out of State Headwaters dnst to SR0030 39.853802 -77.288300 11.12 Adams McDowells Run Carbaugh Run Headwaters to Mouth 39.876610 -77.448990 1.03 Adams Opossum Creek Conewago Creek Headwaters to Mouth 39.931667 -77.185555 12.10 Adams Stillhouse Run Conococheague Creek Headwaters to Mouth 39.915470 -77.467575 1.28 Adams Toms Creek Out of State Headwaters to Miney Branch 39.736532 -77.369041 8.95 Adams UNT to Little Marsh Creek (RM 4.86) Little Marsh Creek Headwaters to Orchard Road 39.876125 -77.384117 1.31 Allegheny Allegheny River Ohio River Headwater dnst to conf Reed Run 41.751389 -78.107498 21.80 Allegheny Kilbuck Run Ohio River Headwaters to UNT at RM 1.25 40.516388 -80.131668 5.17 Allegheny Little Sewickley Creek Ohio River Headwaters to Mouth 40.554253 -80.206802 -
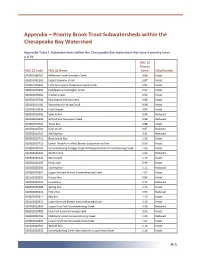
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

West Branch Subbasin Survey 2015
West Branch Susquehanna River Subbasin Year 1 Survey, April through August 2015 Pub. No.308 September 2016 Ellyn J. Campbell Supervisor, Monitoring and Assessment Susquehanna River Basin Commission TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 1 METHODS USED IN THE 2015 SUBBASIN SURVEY ............................................................. 5 Long-term Sites ........................................................................................................................... 6 Probabilistic Sites........................................................................................................................ 6 Water Chemistry and Discharge ................................................................................................. 8 Macroinvertebrates ................................................................................................................... 10 Physical Habitat ........................................................................................................................ 11 RESULTS and DISCUSSION ...................................................................................................... 11 CONCLUSIONS........................................................................................................................... 25 REFERENCES ............................................................................................................................. 27 FIGURES -

Journal of the Lycoming County Historical Society, Summer 1967
28 THE JOURNAL S. Duss and his wife, Susie, cook charge of (obtained from some distant neighbor) to affairs, and still continue, though Dubs is keep the cold out at night. In the morning very old. they found their window gone, and upon looking out saw some deer were walking The community of Economy prospered until their estimatedwealth became twenty away af ter having eaten then: straw. millions. When the original leadersdied the Doctor Holler during his winter in Ger- oi:ganization became corrupt and is now mantown had become a Dunker. and al- rapidly disintegrating though his company were reformed Luther- ans (bur nicknamed Pietists) they were In the colony at Economy were relatives of settlers in blooming Grove, and one of easily persuaded to accept this form of fain) them, Samuel Hendricks, came occasionally, and practice which was an easy transition, so that they came to Lycoming county when an old than, to visit among his friends, known as Dunkers. and, as though uneasy as to the disposal of his estateafter his death.wanted his kind- red to visit him in order to establisha claim. O]UGINAL COLONISTS None would go, until after he had passed The colony of 1804 comprisedthe f allow away, when on [wo occasions about 1890, SamuelGoetz and Conrad Solomon wcnt to ing named persons: John and Gottlieb Heim, Leonard Ulmer and family, Leonard rhe community authorities to claim their Steigerand fatnily, John GeorgeWaltz and inheritance.After being royally entertained family, John GeorgeKiess and family, Da- f or a week, they were presentedwith a bot- vid Young and family, Wendel Harmon and tle of wine, tickets for their return and po- family, Michael Gross and family, Michael litely conductedto the Crainfor Williams- Diehl and family, Ford. -

State Game Lands 114
77°17'30"W 77°15'0"W 77°12'30"W 77°10'0"W 77°7'30"W ROAD CLASSIFICATION Secondary Highway Unimproved Road ! Electric Oil Pipeline; Gas Line Other Line Phone Sewer Line; Water Line Trail !! Special Trails Stream IAl IA Parking Area ²³F Food & Cover Crew HQ ²³G Garage L Headquarters ²³O Other ²³S Storage l Gate YYY Tower Site 41°22'30"N Food Plot 41°22'30"N Featured Game Lands Adjacent Game Lands Wetland PENNSYLVANIA GAME COMMSISSION STATE GAME LANDS 41°20'0"N 114 41°20'0"N LYCOMING COUNTY Feet 0 2400 4800 7200 9600 REVISED July 2016 Copyright:©Copyright: © 20132014 PENNSYLVANIA National Geographic GAME COMMISSION Society, i-cubed 77°17'30"W 77°15'0"W 77°12'30"W 77°10'0"W 77°7'30"W State Game Lands 114 (2,881.8 acres) is located in Lycoming County 9/29/2011 SPORTSMEN'S RECREATION MAP within the Pennsylvania Game Commission's Northcentral Region and Wildlife Management Unit 2G. The game lands is managed in two compartments and has very limited access due to being bordered by large private hunting clubs and a municipal water authority. Compartment 1 has the only public access off of Township Route 784 and Compartment 2 is only accessible by foot through the water authority property. Topography is mostly very steep side hills. The Highest elevation of 1814 feet is found on the ridge tops between Watt Hollow and Larry's Creek in Compartment 1. The lowest elevation of 940 feet is found along Larry's Creek where it leaves the Game Lands in Compartment 2. -

Lycoming County Chesapeake Bay Tributary Strategy
Lycoming County’s Implementation Plan For the Chesapeake Bay Tributary Strategy The Lycoming County Conservation District’s Board of Directors approved this version of the Lycoming County Implementation Plan for the Chesapeake Bay Tributary Strategy during their February18, 2015 meeting. 1 Table of Contents County Description……………………………………………………………………………….Page 3 Past Accomplishments Impaired Waters of Lycoming County…………………………………………….......................Page 5 US EPA Priority Agricultural Streams Impaired by High Total Nitrogen……………...………..Page 8 USDA-NRCS Priority Watersheds Priority Areas Map of Lycoming County’s Impaired Water…………………………………………………...Page 10 Technical Resource……………………………………………………………….......................Page 11 Funding Source Best Management Practices Agricultural Compliance…...…………………………………………………………………...Page 12 Agricultural Land Preservation Programs and Long Term Easement Programs Barnyard Runoff Control………………………………………………………….......................Page13 Conservation Plans Cover Crops……………………………………………………………………………………..Page 14 Dirt and Gravel Road Pollution Prevention Program Managed Precision Agriculture No-till Farming Nutrient Management Planning………………………………………………………………....Page 15 Nutrient Trading…………………………………………………...……………………………Page 16 Public Education Stream Bank Fencing, Off Stream Watering Systems and Riparian Forest Buffers……………Page 17 Stream Bank Stabilization and Stream Bank Restoration………………………........................Page 18 Storm Water Management…………………………………………...………………………….Page 19 Urban Nutrient Management -

Class a Wild Trout Waters Created: August 16, 2021 Definition of Class
Class A Wild Trout Waters Created: August 16, 2021 Definition of Class A Waters: Streams that support a population of naturally produced trout of sufficient size and abundance to support a long-term and rewarding sport fishery. Management: Natural reproduction, wild populations with no stocking. Definition of Ownership: Percent Public Ownership: the percent of stream section that is within publicly owned land is listed in this column, publicly owned land consists of state game lands, state forest, state parks, etc. Important Note to Anglers: Many waters in Pennsylvania are on private property, the listing or mapping of waters by the Pennsylvania Fish and Boat Commission DOES NOT guarantee public access. Always obtain permission to fish on private property. Percent Lower Limit Lower Limit Length Public County Water Section Fishery Section Limits Latitude Longitude (miles) Ownership Adams Carbaugh Run 1 Brook Headwaters to Carbaugh Reservoir pool 39.871810 -77.451700 1.50 100 Adams East Branch Antietam Creek 1 Brook Headwaters to Waynesboro Reservoir inlet 39.818420 -77.456300 2.40 100 Adams-Franklin Hayes Run 1 Brook Headwaters to Mouth 39.815808 -77.458243 2.18 31 Bedford Bear Run 1 Brook Headwaters to Mouth 40.207730 -78.317500 0.77 100 Bedford Ott Town Run 1 Brown Headwaters to Mouth 39.978611 -78.440833 0.60 0 Bedford Potter Creek 2 Brown T 609 bridge to Mouth 40.189160 -78.375700 3.30 0 Bedford Three Springs Run 2 Brown Rt 869 bridge at New Enterprise to Mouth 40.171320 -78.377000 2.00 0 Bedford UNT To Shobers Run (RM 6.50) 2 Brown -

Ecosystem Flow Recommendations for the Susquehanna River Basin (PDF)
Ecosystem Flow Recommendations for the Susquehanna River Basin Report to the Susquehanna River Basin Commission and U.S. Army Corps of Engineers © Mike Heiner Submitted by The Nature Conservancy November 2010 Ecosystem Flow Recommendations for the Susquehanna River Basin November 2010 Report prepared by The Nature Conservancy Michele DePhilip Tara Moberg The Nature Conservancy 2101 N. Front St Building #1, Suite 200 Harrisburg, PA 17110 Phone: (717) 232‐6001 E‐mail: Michele DePhilip, [email protected] i Acknowledgments This project was funded by the Susquehanna River Basin Commission (SRBC) and U.S. Army Corps of Engineers, Baltimore District (Corps). We thank Andrew Dehoff (SRBC) and Steve Garbarino (Corps), who served as project managers from their respective agencies. We also thank Dave Ladd (SRBC) and Mike Brownell (formerly of SRBC) for helping to initiate this project, and John Balay (SRBC) for his technical assistance in gathering water use information and developing water use scenarios. We thank all who contributed information through workshops, meetings, and other media. We especially thank Tom Denslinger, Dave Jostenski, Hoss Liaghat, Tony Shaw, Rick Shertzer and Sue Weaver (Pennsylvania Department of Environmental Protection); Doug Fischer, Mark Hartle and Mike Hendricks (Pennsylvania Fish and Boat Commission); Jeff Chaplin, Marla Stuckey, and Curtis Schreffler (U.S. Geological Survey Pennsylvania Water Science Center); Stacey Archfield (USGS Massachusetts‐ Rhode Island Water Science Center); Than Hitt, Rita Villella and Tanner -

West Branch Paddle and Pedal Sojourn
Lycoming County Watershed E- Notes April 2015 LCCD Annual Seedling Sale Lycoming County Conservation District: Seedling Sale Brochure and Order Form are now available on the LCCD Website. Pickup date - Friday, April 17, 2015 Noon to 6:00 PM- County Farm Complex Local Cooperative Trout Hatcheries to be topic of April 8 Meeting The roles of our area’s cooperative trout hatchery programs and their benefits to local cold water fishing streams and opportunities will be the topic of presentations at the Wednesday, April 8th meeting of the Susquehanna Chapter of Trout Unlimited at 7pm at the Fellowship Hall at the rear of the First Presbyterian Church, Corner of East Third and Mulberry Streets, Williamsport. The public is welcome and encouraged to attend. Muncy Creek Consolidated Sportsmen and Lycoming Creek Anglers operate nurseries in cooperation with the PA Fish and Boat Commission to help supply our local streams with trout for the angling public, with the work dependent on dedicated volunteers. Please come to hear about their programs and what is involved in raising fish and stocking streams, and how you might be able to help out. Slate Run Tackle Shop has donated a new Orvis Hydros fly line which will be given away as a door prize at the meeting. Please note the time change to 7pm. Fish Habitat Project on Carpenters Run Construction will begin April 13th on a farm property on Carpenters Run. The project will include 10 multi Log vane deflectors, 5 modified cross vanes for fish habitat enhancement, stream bank fencing and a riparian tree planting.