Participant Information Leaflet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Integrated Palliative Care of Respiratory Disease Stephen J
Integrated Palliative Care of Respiratory Disease Stephen J. Bourke • Tim Peel Editors Integrated Palliative Care of Respiratory Disease Second Edition Editors Stephen J. Bourke Tim Peel Department of Respiratory Medicine Policy, Ethics and Life Sciences Royal Victoria Infirmary Newcastle University Newcastle Upon Tyne Newcastle Upon Tyne UK UK Previously published by Springer ISBN 978-3-030-18943-3 ISBN 978-3-030-18944-0 (eBook) https://doi.org/10.1007/978-3-030-18944-0 © Springer Nature Switzerland AG 2013, 2019 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, expressed or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. -

Locum Consultant in Cancer Genetics (2 Years Fixed Term)
RECRUITMENT INFORMATION PACK LOCUM CONSULTANT IN CANCER GENETICS (2 YEARS FIXED TERM) CONTENTS PAGE Section A Introduction from Sir Leonard Fenwick CBE, 3 Chief Executive Section B Overview 4 Section C About the Trust 6 Section D About the Area 16 Section E Introduction to the Directorate 17 Section F Advertisement 18 Section G Job Description 20 Section H Person Specification 23 Section I Job Plan 26 Section J How to Apply 28 Section K Main Terms & Conditions of Service 32 Section L Staff Benefits 34 THE NEWCASTLE UPON TYNE HOSPITALS NHS FOUNDATION TRUST SECTION A Introduction from Sir Leonard Fenwick CBE, Chief Executive As one of the largest and highest performing NHS Foundation Trusts in the country, we are unrelenting in our endeavour for clinical excellence, continuously seeking to improve the services we provide for our patients and the communities we serve. The Trust consistently meets the Care Quality Commission (CQC) ‘Essential Standards of Quality and Safety’ which recently confirmed a rating following inspection of ‘Outstanding’. Our services are rated amongst the best in the country according to the Care Quality Commission (CQC) Inpatient Survey 2015; in the most recent NHS Friends and Family Test 98% of our in-patients would recommend our services, and 96% of our staff recommends the patient care provided. We are very proud of our initiatives and improvements in quality of care; while the challenges which remain are greater than ever we are confident that will continue to embrace the opportunities to be innovative and enhance the quality and safety for patients and staff. -

PAUL SCOTT Lives and Works in Cumbria, England, UK
PAUL SCOTT Lives and works in Cumbria, England, UK EDUCATION 2010 PhD., Manchester Institute for Research and Innovation in Art and Design, Manchester Metropolitan University, Manchester, England, UK 1977 B. Ed. Art and Design (2.1.Hons), St. Martin’s College, Lancaster, England, UK 1975 Certificate of Education, St. Martin’s College, Lancaster, England, UK SOLO EXHIBITIONS 2019 Paul Scott, New American Scenery in Raid the Ice Box, Rhode Island School of Design Museum, Providence, RI 2017 Home Truths, PEER London, UK 2016 Cuttings, Ruins, Refugees and Wild Roses, Scottish Gallery, Edinburgh, Scotland Trees and Cuttings, Cheeseburn Sculpture Park, Northumberland, UK 2015 Scenery – Gardens, Bridges, Trucks, Turbines and Willows, Benson Hall Gallery, Providence, Rhode Island, USA, with Andrew Raftery Confected Borrowed and Blue, Bowes Museum, Barnard Castle, Teesdale, England Confected Borrowed and Blue, Harley Gallery, Nottinghamshire, England Confected Borrowed and Blue, Aberystwyth Arts Centre, Aberystwyth, Wales Confected Borrowed and Blue, The Potteries Museum and Gallery, Stoke-on-Trent, England 2014 Confected Borrowed and Blue, Holburne Museum, Bath Spa, Bath, England, UK 2013 Cumbrian Blue(s), Erie Art Museum, Erie, PA Cumbrian Blue(s), Words by the Water Festival, Theatre by the Lake, Keswick, England, UK KHiB Bergen (with Herbert Wiegand), Rom8, Bergen, Denmark 2012 Scottish Gallery, Edinburgh, Scotland, UK 2011 Blås&knåda, Stockholm, Sweden 2010 Illustrious Wonderers with Kurt Wesier (USA), Stephen Bowers (Australia), and Paul -

Newcastle Hospitals Annual Report and Accounts 2019-20
Annual Report and Accounts 2019/20 Annual Report and Accounts 2019/20 Presented to Parliament pursuant to Schedule 7, paragraph 25 (4) (a) of the National Health Service Act 2006 Contents Chairman and Chief Executive Introduction 6 Our Trust Strategy, Vision and Values 8 Service Developments and Achievements 10 Partnerships 18 Research 22 Awards and Achievements 26 Flourish 32 Charitable Support 34 1. Performance Report 38 A. Overview of performance 38 Our Activities 39 Key risks to delivering our objectives 40 The Trust 42 Going concern 43 Operating and Financial Performance 44 B. Performance report 48 Analysis of Performance 48 Sustainability 58 Health and Safety 64 4 2. Accountability report 66 Board of Directors Audit Committee Better Payments Practice Code and Invoice Payment Performance Income Disclosures NHS Improvement’s Well-Led Framework Annual Statement on Remuneration from the Chairman Annual Report on Remuneration Remuneration Policy Fair Pay Our Governors Governor Elections Nominations Committee Membership Staff Report Code of Governance NHS Oversight Framework Statement of Accounting Officer’s Responsibilities Annual Governance Statement Audit and Controls Abbreviations and Glossary of Terms 3. Annual Accounts 2019/20 Chairman and Chief Executive Introduction Our annual report this year is written This year, we became the first NHS Trust as we begin to emerge from the height and the first health organisation in the of the COVID-19 pandemic and what world to declare a Climate Emergency, has been one of the most challenging committing us to taking clear action to periods in the NHS’s history. On 31 achieve net zero carbon. The significant January 2020, our High Consequence impact of climate change on the health Infectious Disease Unit received the first of the population makes it vitally patients in the UK who were confirmed important for us to take positive action to have the virus, which had been first to preserve the planet. -

Royal Newcastle Infirmary
Accounting for Healthcare in the Newcastle Infirmary During the 19th Century Andrew John Holden Thesis submitted for the degree of Doctor of Philosophy Newcastle University Business School June 2018 i To Gill, Olly and Emily for all your support, encouragement and love ii Newcastle Infirmary c 1815 Figure 0.1 – The Newcastle Infirmary (Source: Welcome Library Images) To serve the needy, sick and lame, This splendid shilling freely came, From one who knows the want of wealth, And what is more - the want of health. Beneath this roof may thousands find, The greatest blessings of mankind; And hence may millions learn to know, That to do good’s our end below; That Vice and Folly must decay Ere we can reach eternal day! (Anon. Above poem written on a note which enclosed a shilling left in a poor box 1752 – from Hume 1951, p. 5) iii Abstract Accounting played a critical role in the management of the Newcastle Infirmary during the 19th century. In a class-based society, the poor relied upon the generosity of the wealthy for their healthcare at a time when poverty itself was seen as a sin, an act against God. These wealthy donors established and maintained hospitals, such as the Newcastle Infirmary, and were responsible for the governance, management and admission of patients. Their aim was to be seen to use resources efficiently and to treat the “deserving poor” to restore them to productive members of society. Throughout the century new buildings, medical advances and increasingly highly specialised staff had to be financed to cope with increasing demand. -

BIA Grant Application Form
www.britishinfection.org BIA Grant Application Form Please complete within the space provided - The form should be submitted electronically by MS word or .pdf document to the Scientific Affairs Secretary at [email protected] Please indicate below the scheme to which you are applying BIA Research Fellowship x BIA Small Research Project Grant BIA Clinical Exchange 1. Applicant (complete section 1A as well if applying for BIA Clinical Exchange or research in an overseas centre): Applicant (details of current work) UK Sponsor Surname Payne Chinnery Forename(s) Brendan Patrick Age 36 Title Dr Prof Post Held Clinical Lecturer in Infectious Diseases Professor of Neurogenetics and Virology Department: Department of Infection and Tropical Institute of Genetic Medicine Medicine Institution: Royal Victoria Infirmary Newcastle University Address: Elliott Building Wellcome Centre for Mitochondrial Research Royal Victoria Infirmary Institute of Genetic Medicine Newcastle-upon-Tyne Central Parkway NE1 4LP Newcastle-upon-Tyne NE1 3BZ Telephone No: 0191 282 1104 0191 241 8611 Fax No: 0191 282 6276 0191 241 8666 Email: [email protected] [email protected] [Type here] 1 BIA\Forms\Grants_submission_form_2014 1A. Overseas Research training and BIA Clinical Exchange applicants ONLY Details should be given of the overseas sponsor and/or supervisor. Applicant (details of current work) Sponsor (overseas institution) Surname Forename(s) Age Title Post Held Department: Institution: Address: Telephone No: Fax No: Email: 2 2a) Institution/Authority (administering grant if approved): Newcastle University (will administer grant) Newcastle-upon-Tyne Hospitals NHS Foundation Trust (co-applicant) b) Address at which work is to be done: Royal Victoria Infirmary, Newcastle-upon-Tyne, and Institute of Genetic Medicine, Newcastle University 3. -

Global Innovation in the Centre of Newcastle a Landmark 24-Acre Quarter Built to Transform Quality of Life with New Products and Services
Global innovation in the centre of Newcastle A landmark 24-acre quarter built to transform quality of life with new products and services 01 The Helix is Newcastle’s flagship development and the only city-centre quarter of its kind in the UK. Hundreds of innovators, businesses and progressive homeowners living and working side by side, along with great food, drink and entertainment venues and three beautiful new public spaces. But it’s so much more than a collection of cutting-edge buildings. It’s a 24-acre testbed and collaborative ecosystem for public and private bodies. We have carefully brought together world-class researchers, buzzing startups and international brands and actively help them collaborate to bring brilliant innovations to the marketplace. Newcastle Helix is a hub for businesses and academics at the leading edge of data science, urban science and life science. Together, we’re transforming the quality of life for families, communities and cities around the world. And that’s what living better is all about. Explore Newcastle Helix 02 0003 One of Europe’s most exciting innovation districts Newcastle Helix comprises 10 world-class buildings covering 500,000 sq ft, united by our vision for better living. It’s a unique ecosystem, purpose-built to enable the commercialisation of your company’s new ideas. By providing access to on-site corporates, SMEs, research hubs, National Innovation Centres and Newcastle University, the Helix helps accelerate your products and services to market. It’s the only city-centre ecosystem of its kind in the UK, with a catchment of complementary specialisms to support the full breadth of data, life, and urban sciences. -

Recruitment Information Pack
Recruitment Information Pack LOCUM CONSULTANT INTERVENTIONAL RADIOLOGIST AUGUST 2019 careers.nuth.nhs.uk @NewcastleHosps facebook.com/NewcastleHosps Content Page Section A Introduction 3 Section B Overview 4 Section C About the Trust 5 Section D About the Area 14 Section E Advert 15 Section F Job Description 16 Section G Person Specification 18 Section H Job Plan 20 Section I Main Terms & Conditions of Service 22 Section J Additional Information 23 2 Section A Introduction from Dame Jackie Daniel, Chief Executive Officer The Newcastle upon Tyne Hospitals NHS Foundation Trust is a hugely successful organisation, with highly skilled staff, dedicated to providing the best possible care for the people of the North East and beyond. As one of the largest and highest performing NHS Foundation Trusts in the country, we are continuously seeking to improve our services including having among the highest number of specialist services of any Trust in the UK. We are, of course, proud to have been acknowledged in 2016 as ‘Outstanding’ by the Care Quality Commission. Operating across multiple locations (Freeman Hospital, Royal Victoria Infirmary, Campus for Ageing and Vitality and Centre for Life) and a number of community sites, our services are rated amongst the best in the country according to the Care Quality Commission (CQC) Inpatient Survey 2017; in the most recent NHS Friends and Family Test around 98% of our in-patients would recommend our services, and 96% of our staff recommends the patient care provided. We form a key part of one of Europe’s leading centres for research and innovation with formal management relationships with both Newcastle University and the University of Northumbria in Newcastle and a high profile with the National Institute of Health Research. -
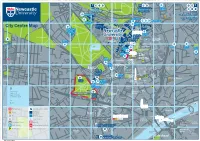
Campus for R Infirmary (RVI) O E Y a a Parade D 1 C R a KING’S WK G D O 6 Ageing K D N a I 7 I R
Nuns Moor OSBORNE ROAD GREAT NORTH RD C LA 78 94 95 85 86 84 87 88 FENHAM HALL DRIVE RE Exhibition Park MO NT A167(M) to: Great North Road to: Easton RD 89 90 91 St Mary’s College A1 North Bowsden Court Flats Cockle Park Farm Newcastle International Airport Freeman Hospital A1058 to: AD Nafferton Farm Heaton Sports Ground Hunter’s Moor RO R’S 71 Marris House Cochrane Park Sports Ground TE CE ( N NTRAL ORWAY A167 M) PORTLAND TER Henderson Hall U MOT JESMOND ROAD A1058 Nuns Moor H Longbenton Sports Ground Park Terrace Jesmond O 73 CLAREMONT RD S Dove Marine Laboratory Windsor Terrace B 45 44 43 42 O R Richardson N Kensington Road E 46 Terrace T Faculty of E Medical Sciences CL R City Centre Map 75 AR A E Robinson 1 M VE 8 Castle Leazes O Library C O 9 N C ED GR T E AR including N RO OR ROAD ON R PORTLAND ROAD AD S MO HT Newcastle D 36 T N Castle Court RICHARDSON ROAD R NU IG S R A TU B Jesmond L D LEY Great North M T Road O E University D RR Museum: T A R O C E Nuns Moor Park R Hancock D W 77 Hatton R Northumbria B A D Northern O A Royal Victoria Y R Leazes A Gallery F University Stage Campus for R Infirmary (RVI) O E Y A A Parade D 1 C R A KING’S WK G D O 6 Ageing K D N A I 7 I R W R Civic A A R 92 L O R S ( 83 76 ’ S and Vitality T M ER A G B T D O Centre ER T N S ) R I S A C K A Victoria Barker C T E I 9 R Leazes Park O V House Turner Boating Lake Bernicia R Hall D A N N Halls D Court EW E B M 12 A ILL E ACE S ’S PL R U T MARY S T 101 Q Joseph Cowen S Arthurs City Hall T Halls R 14 City E Hill NORTHUMBERLAND RD E Haymarket T Leazes L E N Centre BRIGHTON GROVE Terrace A O Z Haymarket Newcastle R E T S Haymarket General 79 H U J T P Bus Station Hospital W PERCY STREET O E Albion A Eldon Garden M E H BEACONSFIELD STREET E R B N R L N A T K Shopping Centre R House E R T L DU R D S I N Newcastle Utd R ERR L O SSLEY T ACE O R Northumbria E RO STANHOPE STREET G Football Club 98 A D C B A C T B N University A D Eldon Square S O R N R D O A Newcastle E N C Bus Station and K S N R City R S O University T Shopping Centre S A T AD . -

Presenting Features and Early Management of Childhood Intermittent Exotropia in the UK
Presenting Features and Early Management of Childhood Intermittent Exotropia in the UK: Inception Cohort Study Deborah Buck, Christine Powell, Phillipa Cumberland, Robert Taylor, John Sloper, Peter Tiffin, Helen Davis, Jugnoo Rahi, Michael P Clarke To cite this version: Deborah Buck, Christine Powell, Phillipa Cumberland, Robert Taylor, John Sloper, et al.. Pre- senting Features and Early Management of Childhood Intermittent Exotropia in the UK: Inception Cohort Study. British Journal of Ophthalmology, BMJ Publishing Group, 2009, 93 (12), pp.1620-n/a. 10.1136/bjo.2008.152975. hal-00486085 HAL Id: hal-00486085 https://hal.archives-ouvertes.fr/hal-00486085 Submitted on 25 May 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Title page Presenting Features and Early Management of Childhood Intermittent Exotropia in the UK: Inception Cohort Study Deborah Buck1, Christine Powell2, Phillipa Cumberland3, Helen Davis4, Emma Dawson5, Jugnoo Rahi3, John Sloper5, Robert Taylor6, Peter Tiffin7, Michael Clarke2,8. On behalf of the Improving Outcomes in Intermittent -
The Magazine of the University of Newcastle Upon Tyne
ARCHESThe Magazine of the University of Newcastle upon Tyne Issue 5 | Autumn 2004 Making Art in the Middle East Challenges of an Ageing Society Gaining the Competitive Advantage ARCHES Editorial Welcome to the latest issue of Arches magazine At the heart of Newcastle University’s mission is the ambition to be a world-class research-intensive institution. We have a proud research record here at Newcastle and a history within the North-East region that boasts inventions of truly international significance – just imagine a world without Joseph Swann’s electric light bulb! It is therefore particularly gratifying to observe that our work in science and technology continues to push back the frontiers of knowledge and understanding, and to catch the imagination of the world in the process. In August, Newcastle made international headlines on an unprecedented scale with the decision of the Human Fertilisation and Embryology Authority (HFEA) to grant a licence to our Newcastle Human Embryonic Stem Cell Group to undertake cloning of human embryos, or ‘somatic cell nuclear transfer’ as it is known scientifically. The Group was established two years ago, in an innovative joint venture involving the University. Its remit is to explore the potential offered by stem cells in order to understand and develop possible new therapies for many serious and debilitating diseases. In early 2003 it became one of the first two groups in the UK to derive human ES (embryonic stem) cells from spare IVF embryos, leading to the licence application to HFEA in February 2004. This is the first time in the UK that such a licence has been granted. -
Campus Map 61 N 50 N
University Information Taxi TravelShop Airport Metro Toilet Railway Station Baby Change Hospital Accessible Toilet Bus Parking R I D M C A OR H O P A E RR TH S D ST A167 towards ’ S R R EE E O T A1 North T N N R U O Newcastle International Airport H A D Bowsden Court BRANDLING PK Freeman Hospital C LA 84 85 86 RE MO Exhibition Park Great North Road to: NT 67 RO Easton Flats AD Bowsden Court Marris House 66 Freeman Hospital 71 78 94 95 ET MOTORWAY A167(M E L ) RE B Student Flats 68 North Terrace 92 CENTRA ST E C A167(M) to: M L A RU L R PG Houses C E 70 Claremont Road to: St Mary’s College N R A G E R T Sports Moorbank Botanical Cockle Park Farm O E O V V Gardens Nafferton Farm E X O Centre N W 65 W R A A G E L M L 56 S E A C T E C L C 69 L AC R L E A R E R R E ST Claremont Place E TE 45 44 S B R 55 M K C 72 E F O R 43 E E R PG House A T A N P K N M T L 54 E T ING R T O N ON 53 A 42 F P D S Windsor Terrace O LA I CE 57 N U 73 Richardson Road G Student Flats N 63 T T 46 A O WINDSOR TERRACE I Campus Map 61 N 50 N R 60 62 52 T O E 47 W 38 51 R 37 39 R 40 LOVERS’ L 64 AN A E C 41 59 E E IV C 49 DEVONSHIRE TER Robinson R L D A Jesmond D R 48 Library N A E Road LL M O 35 H 75 R 58 24 O DEVONSHIRE WALK 36 I N T C T ES Leazes Park H Claremont W Castle Leazes A 33 34 D R Quad OA and Castle Court D Great R R D S Percy ON O RVI: Leazes Wing Quad O North M 83 N 32 A ES Old 25 D Museum: J SANDYFORD ROAD R 23 E Sandyford Road to: O CLAREMONT WALK Hancock G A D Library Victoria Hall D A D 31 I O 26 R R 28 87 88 89 90 91 B A S 74 I A1058 to: A R OLD QUAD