Pterois Miles) Into the Mediterranean Sea
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Documenting the Invasion of Lionfish in Florida's Waters And
Documenting the Invasion of Lionfish in Florida’s Waters and Management Efforts to Control Them Image credit: Image Bryan Fluech Scorpionfish Family Two visually identical species found in the Southeastern U.S. Red Lionfish (Pterois volitans) Devil Firefish (Pterois miles) Image credits: www.lionfishhunters.org Native Distribution Red Lionfish – Pterois volitans Devil Firefish – Pterois miles Location of Venomous Spines 13 2 3 Venomology Image Image credit: REEF Lionfish venom is a protein based neurotoxin and is contained in glandular venom tissue in grooves along either side of each spine Image credit: Dawn Witherington Lionfish Envenomation Pain is immediate; intensifies “It won’t kill you, but it’ll over 60-90 minutes; may last make you wish you were 6-12 hours dead.” Treatment involves covering the wound with hot (not scalding) water; use of over-the-counter pain relievers Victims should seek medical attention Other symptoms might include ulceration of the wound site, headaches, nausea or diarrhea Image Credit: Roxane Boonstra Lionfish Invasion First record : 1985 in Dania, FL Aquarium releases or escapes are most likely sources of the invasion Over 60,000 imported into Florida annually* Mitochondrial data show no evidence of multiple independent introductions Documenting the Invasion Image credit: USGS Lionfish Issues Image credit: Florida Sea Grant Highly Productive Year round reproduction Can spawn every 2-4 days 20,000 – 30,000 eggs / spawn Larvae dispersed by currents Sexually mature within a year Egg Mass Image -

The Invasion of Indo-Pacific Lionfish in the Bahamas: Challenges for a National Response Plan
The Invasion of Indo-Pacific Lionfish in the Bahamas: Challenges for a National Response Plan KATHLEEN SULLIVAN SEALEY1, 4, LAKESHIA ANDERSON2, DEON STEWART3, and NICOLA SMITH4, 5 1University of Miami Department of Biology, Coral Gables, Florida USA; 2 Department of Marine Resources, Nassau, Bahamas; 3 Bahamas Environment, Science and Technology Commission, Nassau, Bahamas; 4 Marine and Environmental Studies Institute, College of The Bahamas, Nassau, Bahamas; 5 Department of Zoology, University of British Columbia, Vancouver, BC, Canada ABSTRACT The invasion of the Indo-Pacific lionfish to Bahamian waters raises considerable concern due to the uncertainty of its ecological impacts and its potential threats to commercial fisheries and human safety. Lionfish have been reported throughout the archipelago and are the focus of several research and monitoring initiatives. The Bahamas has a National Invasive Species Strategy, but marine invasions require unique partnerships for small islands developing states to develop realistic management goals and actions. The Government of The Bahamas has limited funds to address major resource management issues; hence, collaboration with non-governmental agencies and tertiary education institutions is imperative. The establishment and spread of lionfish has created a novel opportunity for the formation of innovative public-private partnerships to address the ecological, economic and social impacts of biological invasions. KEY WORDS: Lionfish, invasion, reefs La Invasión en las Bahamas del Pez León del Indo-Pacifico: Un Caso de Investigación, Planificación, y Manejo La invasión del pez león del Indopacífico en las aguas de Las Bahamas ha generado una gran preocupación debido a la incertidumbre sobre su efecto ecológico y la posible afectación a la pesca comercial, el turismo y la seguridad de la población. -

Feeding Ecology of Invasive Lionfish (Pterois Volitans) in the Bahamian Archipelago
Environ Biol Fish (2009) 86:389–398 DOI 10.1007/s10641-009-9538-8 Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian archipelago James A. Morris Jr. & John L. Akins Received: 24 February 2009 /Accepted: 7 October 2009 /Published online: 27 October 2009 # US Government 2009 Abstract Feeding ecology of the lionfish (Pterois Keywords Pterois . Diet composition . volitans), an invasive species in the Western North Stomach content . Invasive species Atlantic, was examined by collecting stomach content data from fishes taken throughout the Bahamian archipelago. Three relative metrics of prey quantity, Introduction including percent number, percent frequency, and percent volume, were used to compare three indices The lionfishes, Pterois miles and P. volitans, (Hamner of dietary importance. Lionfish largely prey upon et al. 2007; Morris 2009) are the first non-native teleosts (78% volume) and crustaceans (14% volume). marine fishes to become established along the Twenty-one families and 41 species of teleosts were Atlantic coast of the U.S. and the Caribbean. Adult represented in the diet of lionfish; the top 10 families of lionfish specimens are now found along the U.S. East dietary importance were Gobiidae, Labridae, Gram- Coast from Cape Hatteras, North Carolina, to Florida, matidae, Apogonidae, Pomacentridae, Serranidae, and in Bermuda, the Bahamas, and throughout the Blenniidae, Atherinidae, Mullidae, and Monacanthi- Caribbean, including the Turks and Caicos, Haiti, dae. The proportional importance of crustaceans in the Cuba, Dominican Republic, Puerto Rico, St. Croix, diet was inversely related to size with the largest Belize, and Mexico (Schofield et al. 2009). The first lionfish preying almost exclusively on teleosts. -

Red Lionfish
N. Carolina Aquarium at Ft. Fisher N. Carolina Aquarium at Ft. Fisher COMMON NAME: Red lionfish SCIENTIFIC NAME: Pterois volitans (Linnaeus 1758) NATIVE DISTRIBUTION: Western Pacific from southern Japan to Micronesia, Australia and the Philippines; also throughout most of Oceania (including the Marshall Islands, New Caledonia and Fiji) east to French Polynesia. U.S. distribution: Established along the Atlantic coast from southern Florida to New York, including Bermuda. Habitat: The lionfish inhabits reefs from about 10 to 175 m deep. As juveniles, lionfish live in small groups, but as adults they typically occur alone. Individuals are relatively inactive during the day, typically sheltering U.S. Geological Survey Geological U.S. in reef crevices. Life cycle: Red lionfish are external fertilizers that produce a pelagic egg mass following a courtship and mating process that is not well documented. Like many reef fishes, red lionfish larvae are planktonic. After a few weeks in the plankton stage, larvae settle onto reefs as juveniles. Cool facts: • The red lionfish is a solitary predator of small fishes, shrimps and crabs. • Prey are stalked and cornered or made to feel so by the outstretched and expanded pectoral fins of the red lionfish in full ambush mode. • Prey are ultimately obtained with a lightning-quick snap of the jaws and swallowed whole. • Cannibalism has been observed for this species. • Unlike most scorpionfish with their camouflage markings, the lionfish has greatly extended fin spines and striking colors. Pathways of invasion: Aquarium releases and pet fish liberated during storm events such as hurricanes. AQUATIC INVADERS A Sea Grant/AZA Partnership 1 RED LIONFISH Pterois volitans Impacts: The introduction of the red lionfish to marine waters of the Atlantic off the eastern coast of the U.S. -

Modeling Suitable Habitat of Invasive Red Lionfish Pterois Volitans (Linnaeus, 1758) in North and South America’S Coastal Waters
Aquatic Invasions (2016) Volume 11, Issue 3: 313–326 DOI: http://dx.doi.org/10.3391/ai.2016.11.3.09 Open Access © 2016 The Author(s). Journal compilation © 2016 REABIC Research Article Modeling suitable habitat of invasive red lionfish Pterois volitans (Linnaeus, 1758) in North and South America’s coastal waters 1 1, 2 3 Paul H. Evangelista , Nicholas E. Young *, Pamela J. Schofield and Catherine S. Jarnevich 1 Natural Resource Ecology Laboratory, Colorado State University, Fort Collins, CO 80523, USA 2 US Geological Survey, Gainesville, FL 32653, USA 3 US Geological Survey, Fort Collins Science Center, Fort Collins, CO 80526, USA *Corresponding author E-mail: [email protected] Received: 9 October 2015 / Accepted: 11 April 2016 / Published online: 5 May 2016 Handling editor: Charles Martin Abstract We used two common correlative species-distribution models to predict suitable habitat of invasive red lionfish Pterois volitans (Linnaeus, 1758) in the western Atlantic and eastern Pacific Oceans. The Generalized Linear Model (GLM) and the Maximum Entropy (Maxent) model were applied using the Software for Assisted Habitat Modeling. We compared models developed using native occurrences, using non-native occurrences, and using both native and non-native occurrences. Models were trained using occurrence data collected before 2010 and evaluated with occurrence data collected from the invaded range during or after 2010. We considered a total of 22 marine environmental variables. Models built with non-native only or both native and non-native occurrence data outperformed those that used only native occurrences. Evaluation metrics based on the independent test data were highest for models that used both native and non-native occurrences. -

A Guide to Harmful and Toxic Creatures in the Goa of Jordan
Published by the Royal Marine Conservation Society of Jordan. P. O. Box 831051, Abdel Aziz El Thaalbi St., Shmesani 11183. Amman Copyright: © The Royal Marine Conservation Society of Jordan Reproduction of this publication for educational and other non- commercial purposes is authorized without prior written approval from the copyright holder provided the source is fully acknowledged. ISBN: 978-9957-8740-1-8 Deposit Number at the National Library: 2619/6/2016 Citation: Eid, E and Al Tawaha, M. (2016). A Guide to Harmful and Toxic Creature in the Gulf of Aqaba of Jordan. The Royal Marine Conservation Society of Jordan. ISBN: 978-9957-8740-1-8. Pp 84. Material was reviewed by Dr Nidal Al Oran, International Research Center for Water, Environment and Energy\ Al Balqa’ Applied University,and Dr. Omar Attum from Indiana University Southeast at the United State of America. Cover page: Vlad61; Shutterstock Library All photographs used in this publication remain the property of the original copyright holder, and it should not be reproduced or used in other contexts without permission. 1 Content Index of Creatures Described in this Guide ......................................................... 5 Preface ................................................................................................................ 6 Part One: Introduction ......................................................................................... 8 1.1 The Gulf of Aqaba; Jordan ......................................................................... 8 1.2 Aqaba; -

Invasive Red Lionfish Pterois Volitans Blow Directed Jets of Water at Prey Fish
Vol. 448: 1–5, 2012 MARINE ECOLOGY PROGRESS SERIES Published February 23 doi: 10.3354/meps09580 Mar Ecol Prog Ser OPENPEN ACCESSCCESS FEATURE ARTICLE: NOTE Invasive red lionfish Pterois volitans blow directed jets of water at prey fish Mark A. Albins1,*, Patrick J. Lyons2 1Department of Zoology, Oregon State University, 3029 Cordley Hall, Corvallis, Oregon 97331, USA 2Department of Ecology and Evolution, SUNY at Stony Brook, 650 Life Sciences Building, Stony Brook, New York 11794, USA ABSTRACT: Field and laboratory observations of feeding by invasive Pacific red lionfish Pterois voli- tans were conducted during June through August of 2008, 2009 and 2010 near Lee Stocking Island, Bahamas. Observations of this invasive marine predator revealed a previously undocumented pis- civorous behavior. While slowly approaching prey fish, lionfish produce jets of water directed toward their prey. These jets may confuse or distract prey, and often result in prey fish facing the attacking lion- fish, increasing the probability of head-first capture and swallowing. While a variety of fishes are re - ported to create directed water jets, to our knowl- edge, this is the first report of a fish that does so during the capture of fish prey. This behavior may confer a high degree of predatory efficiency, and thus con- tribute to the dramatic success of this Pacific invader of tropical Western Atlantic and Caribbean coral reefs. KEY WORDS: Lionfish · Invasive species · Predation · Pacific lionfish Pterois volitans in a seagrass bed in the Piscivory · Prey naïveté · Marine fishes Bahamas. Resale or republication not permitted without Photo: Timothy J. Pusack, Oregon State University written consent of the publisher INTRODUCTION may confer a high degree of predatory efficiency and contribute to the dramatic success of this invasion. -

Fish Biodiversity Changes in the Central Mediterranean
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Digital.CSIC Azzurro E.1*, Soto S.2, Garofalo G.3, Maynou F.2 Fistularia commersonii in the Mediterranean Sea: Invasion history and distribution modeling based on presence-only records 1ISPRA, National Institute for Environmental Protection and Research, Piazzale dei Marmi 2, 57128 Livorno, Italy 2Institut de Ciències del Mar (CSIC) Passeig Marítim de la Barceloneta, 37-49, E-08003 Barcelona, Spain 3IAMC-CNR, Institute for Coastal Marine Environment, Via L. Vaccara 61, 91026 Mazara del Vallo, Italy Running title: Fistularia commersonii in the Mediterranean Sea 1 Author to whom correspondence should be addressed. email: [email protected] Abstract The bluespotted cornetfish (Fistularia commersonii) (Osteichtyes, Fistulariidae) is considered to be one of the most invasive species of the Mediterranean Sea and Europe but only scattered information exists on its distribution and abundance. Here we collated the available species records, following its first detection in the Mediterranean Sea, in January 2000, until October 2011. A total of 191 observations were used to reconstruct the invasion sequence, to provide estimates of the rate of spread and to construct an environmental suitability model based on six biophysical variables and the maximum entropy approach (MaxEnt). The results showed that colonization of the Mediterranean Sea proceeded in parallel along the southern and northern rim of the Basin at speeds that reached 1000- 1500 km · yr-1 with a clear decrease in the rate of spread at the Sicily Strait. The most important explanatory variables for describing the distribution of F. -
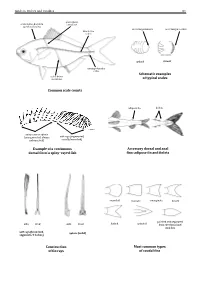
Field Identification Guide to the Living Marine Resources in Kenya
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -
![Pterois Volitans [Linnaeus 1758] and P. Miles [Bennett 1828]) in the Western North Atlantic and Caribbean Sea](https://docslib.b-cdn.net/cover/4030/pterois-volitans-linnaeus-1758-and-p-miles-bennett-1828-in-the-western-north-atlantic-and-caribbean-sea-1694030.webp)
Pterois Volitans [Linnaeus 1758] and P. Miles [Bennett 1828]) in the Western North Atlantic and Caribbean Sea
Aquatic Invasions (2009) Volume 4, Issue 3: 473-479 DOI 10.3391/ai.2009.4.3.5 © 2009 The Author(s) Journal compilation © 2009 REABIC (http://www.reabic.net) This is an Open Access article Research article Geographic extent and chronology of the invasion of non-native lionfish (Pterois volitans [Linnaeus 1758] and P. miles [Bennett 1828]) in the Western North Atlantic and Caribbean Sea Pamela J. Schofield US Geological Survey, Florida Integrated Science Center, Gainesville, FL 32653, USA Email: [email protected] Received 17 July 2009; accepted in revised form 19 August 2009; published online 1 September 2009 Abstract The Indo-Pacific lionfishes (Pterois volitans [Linnaeus 1758] and P. miles [Bennett 1828]: Family Scorpaenidae) are the first non-native marine fishes to establish in the Western North Atlantic. The chronology of the invasion is reported here using records from the US Geological Survey’s Nonindigenous Aquatic Species database. Currently, lionfish are established off the Atlantic coast of the USA from the Florida Keys to Cape Hatteras (North Carolina), the Great Antilles, Bermuda, Bahamas, Cayman Islands and Turks and Caicos. The species have been reported from only one island in the Lesser Antilles (St. Croix), but it is not yet established there. Lionfish are established in Mexico, Honduras and Costa Rica. Reports have come from the Gulf of Mexico (Florida), Belize, Panamá and Colombia; although lionfish are not considered established in these localities at this time (August 2009), invasion is likely imminent. Key words: lionfish, Pterois volitans, Pterois miles, non-native marine fishes, Scorpaenidae Introduction and Akins unpubl. data) and have venomous spines that could injure divers. -

Seamap Environmental and Biological Atlas of the Gulf of Mexico, 2017
environmental and biological atlas of the gulf of mexico 2017 gulf states marine fisheries commission number 284 february 2019 seamap SEAMAP ENVIRONMENTAL AND BIOLOGICAL ATLAS OF THE GULF OF MEXICO, 2017 Edited by Jeffrey K. Rester Gulf States Marine Fisheries Commission Manuscript Design and Layout Ashley P. Lott Gulf States Marine Fisheries Commission GULF STATES MARINE FISHERIES COMMISSION FEBRUARY 2019 NUMBER 284 This project was supported in part by the National Oceanic and Atmospheric Administration, National Marine Fisheries Service, under State/Federal Project Number NA16NMFS4350111. GULF STATES MARINE FISHERIES COMMISSION COMMISSIONERS ALABAMA Chris Blankenship John Roussel Alabama Department of Conservation 1221 Plains Port Hudson Road and Natural Resources Zachary, LA 70791 64 North Union Street Montgomery, AL 36130-1901 MISSISSIPPI Joe Spraggins, Executive Director Representative Steve McMillan Mississippi Department of Marine Resources P.O. Box 337 1141 Bayview Avenue Bay Minette, AL 36507 Biloxi, MS 39530 Chris Nelson TBA Bon Secour Fisheries, Inc. P.O. Box 60 Joe Gill, Jr. Bon Secour, AL 36511 Joe Gill Consulting, LLC 910 Desoto Street FLORIDA Ocean Springs, MS 39566-0535 Eric Sutton FL Fish and Wildlife Conservation Commission TEXAS 620 South Meridian Street Carter Smith, Executive Director Tallahassee, FL 32399-1600 Texas Parks and Wildlife Department 4200 Smith School Road Representative Jay Trumbull Austin, TX 78744 State of Florida House of Representatives 402 South Monroe Street Troy B. Williamson, II Tallahassee, FL 32399 P.O. Box 967 Corpus Christi, TX 78403 TBA Representative Wayne Faircloth LOUISIANA Texas House of Representatives Jack Montoucet, Secretary 2121 Market Street, Suite 205 LA Department of Wildlife and Fisheries Galveston, TX 77550 P.O. -

Fish Movement in the Red Sea and Implications for Marine Protected Area Design
Fish Movement in the Red Sea and Implications for Marine Protected Area Design Thesis by Irene Antonina Salinas Akhmadeeva In Partial Fulfillment of the Requirements For the Degree of Master of Science King Abdullah University of Science and Technology Thuwal, Kingdom of Saudi Arabia April, 2021 2 EXAMINATION COMMITTEE PAGE The thesis of Irene Antonina Salinas Akhmadeeva is approved by the examination committee. Committee Chairperson: Prof. Michael L. Berumen Committee Co-Chair: Dr. Alison Green Committee Members: Dr. Darren Coker, Prof. Rusty Brainard 3 COPYRIGHT © April 2021 Irene Antonina Salinas Akhmadeeva All Rights Reserved 4 ABSTRACT Fish Movement in the Red Sea and Implications for Marine Protected Area Design Irene Antonina Salinas Akhmadeeva The Red Sea is valued for its biodiversity and the livelihoods it provides for many. It now faces overfishing, habitat degradation, and anthropogenic induced climate-change. Marine Protected Areas (MPAs) became a powerful management tool to protect vulnerable species and ecosystems, re-establish their balance, and enhance marine populations. For this, they need to be well designed and managed. There are 15 designated MPAs in the Red Sea but their level of enforcement is unclear. To design an MPA it is necessary to know if it will protect species of interest by considering their movement needs. In this thesis I aim at understanding fish movement in the Red Sea, specifically home range (HR) to inform MPA size designation. With not much empirical data available on HR for Red Sea fish, I used a Machine Learning (ML) classification model, trained with empirical literature HR measurements with Maximum Total Length (L Max), Aspect Ratio (AR) of the caudal fin, and Trophic Level as predictor variables.