Bio Covers Again
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Market Cap Close ADV
Market Cap Close ADV 1598 67th Pctl $745,214,477.91 $23.96 225,966.94 801 33rd Pctl $199,581,478.89 $10.09 53,054.83 2399 Listing_ Revised Ticker_Symbol Security_Name Exchange Effective_Date Mkt Cap Close ADV Stratum Stratum AAC AAC Holdings, Inc. N 20160906 M M M M-M-M M-M-M Altisource Asset Management AAMC Corp A 20160906 L M L L-M-L L-M-L AAN Aarons Inc N 20160906 H H H H-H-H H-H-H AAV Advantage Oil & Gas Ltd N 20160906 H L M H-L-M H-M-M AB Alliance Bernstein Holding L P N 20160906 H M M H-M-M H-M-M ABG Asbury Automotive Group Inc N 20160906 H H H H-H-H H-H-H ABM ABM Industries Inc. N 20160906 H H H H-H-H H-H-H AC Associated Capital Group, Inc. N 20160906 H H L H-H-L H-H-L ACCO ACCO Brand Corp. N 20160906 H L H H-L-H H-L-H ACU Acme United A 20160906 L M L L-M-L L-M-L ACY AeroCentury Corp A 20160906 L L L L-L-L L-L-L ADK Adcare Health System A 20160906 L L L L-L-L L-L-L ADPT Adeptus Health Inc. N 20160906 M H H M-H-H M-H-H AE Adams Res Energy Inc A 20160906 L H L L-H-L L-H-L American Equity Inv Life Hldg AEL Co N 20160906 H M H H-M-H H-M-H AF Astoria Financial Corporation N 20160906 H M H H-M-H H-M-H AGM Fed Agricul Mtg Clc Non Voting N 20160906 M H M M-H-M M-H-M AGM A Fed Agricultural Mtg Cla Voting N 20160906 L H L L-H-L L-H-L AGRO Adecoagro S A N 20160906 H L H H-L-H H-L-H AGX Argan Inc N 20160906 M H M M-H-M M-H-M AHC A H Belo Corp N 20160906 L L L L-L-L L-L-L ASPEN Insurance Holding AHL Limited N 20160906 H H H H-H-H H-H-H AHS AMN Healthcare Services Inc. -

Ipo Analysis
IPO ANALYSIS Snap Research on potential upcoming IPOs from Hamilton Lane Bitcoin Investment Trust selected candidate companies. Visterra Braeburn Pharmaceuticals December, 2016 VentureDeal Snap Files S-1 Registration For $3 Billion IPO Quick Take Camera company Snap Inc. (Pending:SNAP) has filed its initial S-1 registration for a $3 billion IPO at an undisclosed valuation. Snap views the camera as the primary use of the mobile device and seeks to monetize its user base primarily through advertising. The company has a short history of rapidly growing revenues, which exceeded $400 million in 2016. Company Venice-based Snap was founded in 2012 by current CEO Evan Spiegel and current CTO Robert Murphy to initially provide users with a smartphone app that enabled them to post pictures that were automatically deleted after a brief time period. The company has since expanded its scope to include chat, video, media that does not become automatically deleted, and a camera integrated into sunglasses. Below is a brief company video on its recently introduced Spectacles product: (Source: Snap YouTube) Snap has raised in excess of $2.6 billion in several private financing rounds from numerous investors including top tier venture capital firms, corporate investors, private equity firms and government-owned investment enterprises. The company claims that 158 million people use its flagship Snapchat each day, creating over 2.5 billion 'Snaps'. Market and Competition Snap operates a largely advertising-driven business model, although it does sell its Spectacles product for $129 each. It seeks to provide advertisers with access to its user base, which has typically centered around a younger demographic, between the ages of 14-30. -

Fidelity® Total Market Index Fund
Quarterly Holdings Report for Fidelity® Total Market Index Fund May 31, 2021 STI-QTLY-0721 1.816022.116 Schedule of Investments May 31, 2021 (Unaudited) Showing Percentage of Net Assets Common Stocks – 99.3% Shares Value Shares Value COMMUNICATION SERVICES – 10.1% World Wrestling Entertainment, Inc. Class A (b) 76,178 $ 4,253,780 Diversified Telecommunication Services – 1.1% Zynga, Inc. (a) 1,573,367 17,055,298 Alaska Communication Systems Group, Inc. 95,774 $ 317,970 1,211,987,366 Anterix, Inc. (a) (b) 16,962 838,941 Interactive Media & Services – 5.6% AT&T, Inc. 11,060,871 325,521,434 Alphabet, Inc.: ATN International, Inc. 17,036 805,292 Class A (a) 466,301 1,099,001,512 Bandwidth, Inc. (a) (b) 34,033 4,025,764 Class C (a) 446,972 1,077,899,796 Cincinnati Bell, Inc. (a) 84,225 1,297,065 ANGI Homeservices, Inc. Class A (a) 120,975 1,715,426 Cogent Communications Group, Inc. (b) 66,520 5,028,912 Autoweb, Inc. (a) (b) 6,653 19,028 Consolidated Communications Holdings, Inc. (a) 110,609 1,035,300 Bumble, Inc. 77,109 3,679,641 Globalstar, Inc. (a) (b) 1,067,098 1,707,357 CarGurus, Inc. Class A (a) 136,717 3,858,154 IDT Corp. Class B (a) (b) 31,682 914,343 Cars.com, Inc. (a) 110,752 1,618,087 Iridium Communications, Inc. (a) 186,035 7,108,397 DHI Group, Inc. (a) (b) 99,689 319,005 Liberty Global PLC: Eventbrite, Inc. (a) 114,588 2,326,136 Class A (a) 196,087 5,355,136 EverQuote, Inc. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

Valuation Multiples by Industry
Valuation Multiples by Industry https://www.eval.tech SIC Sector: (2000-3999) Manufacturing Report Date: 30 June 2021 Country: United States of America (U.S.A.) Industry Valuation Multiples The table below provides a summary of median industry enterprise value (EV) valuation multiples, as at the Report Date. The data is grouped by industry SIC code: EV Multiple Max # Rev EBITDA EBIT TotAss TanAss Food And Kindred Products (2000) 14 2.27 13.23 16.02 1.94 4.15 Canned, Frozen & Preservd Fruit, Veg & Food Specialties 6 3.66 22.55 32.27 2.10 3.51 (2030) Grain Mill Products (2040) 5 2.14 12.88 18.38 1.48 3.04 Sugar & Confectionery Products (2060) 5 2.98 - - 1.97 2.66 Beverages (2080) 12 4.35 19.33 23.19 2.33 4.47 Malt Beverages (2082) 5 1.97 19.88 - - 1.83 Bottled & Canned Soft Drinks & Carbonated Waters (2086) 9 1.84 12.71 12.91 1.47 3.22 Miscellaneous Food Preparations & Kindred Products (2090) 5 3.89 - - 1.87 7.19 Cigarettes (2111) 5 3.33 10.90 11.89 2.30 3.62 Apparel & Other Finishd Prods Of Fabrics & Similar Matl 12 2.26 10.52 12.04 1.56 2.08 (2300) Men’S & Boys’ Furnishgs, Work Clothg, & Allied Garments 7 2.18 27.71 34.50 2.32 2.80 (2320) Household Furniture (2510) 12 0.96 13.72 16.14 1.15 1.68 Paper Mills (2621) 8 1.10 8.36 12.68 0.79 0.92 Newspapers: Publishing Or Publishing & Printing (2711) 5 0.90 22.92 81.50 0.84 1.49 Chemicals & Allied Products (2800) 10 1.85 16.67 17.68 1.58 2.17 Industrial Inorganic Chemicals (2810) 10 1.58 11.32 20.09 0.90 0.92 Plastic Materials, Synth Resins & Nonvulcan Elastomers 12 2.00 15.35 22.76 1.24 2.25 -
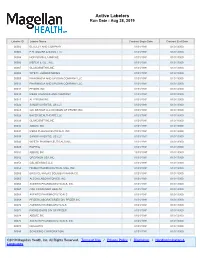
Active Labelers Run Date : Aug 28, 2019
Active Labelers Run Date : Aug 28, 2019 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PHARMACIA AND UPJOHN COMPANY LLC 01/01/1991 01/01/3000 00013 PHARMACIA AND UPJOHN COMPANY LLC 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 GD. SEARLE LLC DIVISION OF PFIZER INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 WYETH PHARMACEUTICALS INC. 01/01/1991 01/01/3000 00049 ROERIG 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 HIKMA PHARMACEUTICAL USA, INC. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. -
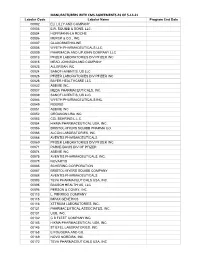
MANUFACTURERS with CMS AGREEMENTS AS of 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY and COMPANY 00003 E.R
MANUFACTURERS WITH CMS AGREEMENTS AS OF 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC Labeler Code Labeler Name Program End Date 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. 00186 ASTRAZENECA PHARMACEUTICALS LP 00187 BAUSCH HEALTH US, LLC. 00206 WYETH PHARMACEUTICALS LLC, 00224 KONSYL PHARMACEUTICALS, INC. 00225 B. F. ASCHER AND COMPANY, INC. 00228 ACTAVIS PHARMA, INC. -

Auslaendische-Aktien-2020.Pdf
Verzeichnis der von der Kursliste der eidg. Steuerverwaltung per 31.12.2020 abweichenden Steuerwerte für die Staatssteuerveranlagung 2020 gilt nur für natürliche Personen mit Domizil im Kanton Basel-Landschaft Steuerwert 31.12.2020 Val. Nr. Titelbezeichnung Titelart in CHF Ausländische Aktien 24582375 ASHTROM GROUP LTD. Namenaktie 15.80 761515 ASICS Corporation Namenaktie 11.50 19531091 ASML Holding NV Inhaberaktie 267.60 828998 AT & S Austria Technologie & Systemtechnik AG Inhaberaktie 21.85 3073309 Aalberts N.V. Inhaberaktie 34.15 903037 Abbott Laboratories Stammaktie 70.30 522413 Abercrombie & Fitch Co. Namenaktie 17.20 903092 Abiomed Inc Namenaktie 143.30 1532828 Acacia Research Corporation Namenaktie 1.75 1184425 Acadia Pharmaceuticals Inc. Namenaktie 23.65 10478724 Accenture plc Namenaktie 160.10 485822 Accor S.A. Inhaberaktie 25.85 812970 Ackermans & van Haaren NV Namenaktie 109.25 3971431 Activision Blizzard, Inc. Namenaktie 47.50 857018 Adacel Technologies Limited Namenaktie 0.52 11730015 Adidas AG Namenaktie 193.60 34149058 Adient plc Namenaktie 15.35 903472 Adobe Inc. Namenaktie 221.05 36416063 Adomos Stammaktie 0.21 36832098 Adtalem Global Education Inc. Namenaktie 15.00 1331600 Advance Auto Parts, Inc. Namenaktie 78.00 903491 Advanced Micro Devices, Inc. Namenaktie 40.55 1027712 Advantech Co., Ltd. Namenaktie 9.55 42119868 Adyen N.V. Stammaktie 1030.20 2736001 Aedifica SA Inhaberaktie 105.85 587479 Aeroflot Namenaktie 0.77 28742001 Aeroporto Guglielmo Marconi di Bologna S.p.A. Namenaktie 8.85 903835 Aflac Incorporated Namenaktie 37.30 36293710 Afterpay Limited Namenaktie 40.25 748779 Agfa-Gevaert N.V. Inhaberaktie 2.15 901692 Agilent Technologies, Inc. Namenaktie 63.50 676894 Agnico Eagle Mines Limited Namenaktie 42.90 42430644 Ahlers AG Namenaktie 0.79 20029778 Ahlstrom-Munksjo Oyj Namenaktie 18.05 18564252 Aimia Inc. -

[ Emc-Lusinnufll'lergcrta'rgets
US 20070255633Al (19) United States (12) Patent Application Publication (10) Pub. N0.: US 2007/0255633 A1 Kridel (43) Pub. Date: NOV. 1, 2007 (54) SYSTEMS AND METHODS FOR INVESTING (52) US. Cl. ....................................................... .. 705/35 (76) Inventor: FIVJigiam J. Kridel, New York, NY (57) ABSTRACT The present invention discloses systems and methods for Correspondence Address? creating and managing ?nancial instruments and indexes PAUL’ HASTINGS’ JANOFSKY & WALKER comprised of securities for companies in subsectors of the LLP economy. These ?nancial instruments alloW investment in R0' BOX 919092 subsectors of the economy Will still being able to minimize SAN DIEGO CA 92191-9092 ’ risk by diversi?cation. The indexes serve as benchmarks for (21) App1_ NO; 11/465,768 companies in the subsectors of the economy. A procedure may be used to identify the securities to include in the (22) Filed: Allg- 18, 2006 ?nancial instruments. This procedure may include (a) iden _ _ tifying securities for companies in a sector of the economy; Related U's‘ Apphcatlon Data (b) limiting the identi?ed securities to those for companies (60) Provisional application No. 60/778,492, ?led on Mar. in a SubSeCIOr Of the SBCIOI‘ Of the economy; (0) applying 1, 2006. focus rules to further limit the identi?ed securities to those _ _ _ _ for companies Who are focused in the subsector of the Pubhcatlon Classl?catlon economy; and (d) limiting the securities included in the (51) Int, Cl, ?nancial instrument or index to those that satisfy other G06Q 40/00 (2006.01) objective criteria. ETF RULE. SET->| APPLICATION’ ' "1 _. -

Companies That Exploit Animals ©2019 Crueltyfreeinvesting.Org Title Stock Exchange Stock Symbol Animal Usage 1-800 FLOWERS.COM, Inc
Companies That Exploit Animals ©2019 CrueltyFreeInvesting.org Title Stock Exchange Stock Symbol Animal Usage 1-800 FLOWERS.COM, Inc. NASDAQ FLWS Meat/Dairy/Eggs 22nd Century Group, Inc AMEX XXII Animal Testing 3M Company NYSE MMM Animal Testing 500.com Limited NYSE WBAI Entertainment|Tangential A. Schulman, Inc. NASDAQ SHLM Animal Testing ABAXIS, Inc. NASDAQ ABAX Animal Testing ABB Ltd NYSE ABB Tangential Abbott Laboratories NYSE ABT Animal Testing|By-product AbbVie Inc. NYSE ABBV Animal Testing Abeona Therapeutics Inc. NASDAQ ABEO Animal Testing Abercrombie & Fitch Company NYSE ANF Leather/Hide/Fur ABIOMED, Inc. NASDAQ ABMD Animal Testing AC Immune SA NASDAQ ACIU Animal Testing Acacia Research Corporation NASDAQ ACTG Animal Testing ACADIA Pharmaceuticals Inc. NASDAQ ACAD Animal Testing Acasti Pharma, Inc. NASDAQ ACST Animal Testing B&G Foods, Inc. NASDAQ BGS Meat/Dairy/Eggs Balchem Corporation NASDAQ BCPC Animal Testing|By-product|Pet Industry Alexion Pharmaceuticals, Inc. NASDAQ ALXN Animal Testing|Meat/Dairy/Eggs Amazon.com, Inc. NASDAQ AMZN Entertainment|Leather/Hide/Fur|Meat/Dairy/Eggs American Airlines Group, Inc. NASDAQ AAL Meat/Dairy/Eggs Amgen Inc. NASDAQ AMGN Animal Testing Bed Bath & Beyond Inc. NASDAQ BBBY Animal Testing|By-product|Leather/Hide/Fur|Tangential Biogen Inc. NASDAQ BIIB Animal Testing C.H. Robinson Worldwide, Inc. NASDAQ CHRW Meat/Dairy/Eggs|Tangential Celgene Corporation NASDAQ CELG Animal Testing Costco Wholesale Corporation NASDAQ COST Leather/Hide/Fur|Meat/Dairy/Eggs DENTSPLY SIRONA Inc. NASDAQ XRAY Animal Testing Dollar Tree, Inc. NASDAQ DLTR Leather/Hide/Fur|Meat/Dairy/Eggs eBay Inc. NASDAQ EBAY Leather/Hide/Fur|Meat/Dairy/Eggs|Pet Industry Endo International plc NASDAQ ENDP Animal Testing Garmin Ltd. -
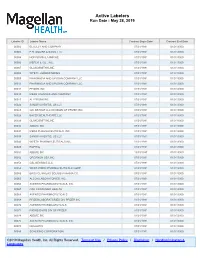
Active Labelers Run Date : May 28, 2019
Active Labelers Run Date : May 28, 2019 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 07/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 07/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 07/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PHARMACIA AND UPJOHN COMPANY LLC 01/01/1991 01/01/3000 00013 PHARMACIA AND UPJOHN COMPANY LLC 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 07/01/1991 01/01/3000 00023 ALLERGAN INC 07/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 07/01/1991 01/01/3000 00025 GD. SEARLE LLC DIVISION OF PFIZER INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 07/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 07/01/1991 01/01/3000 00046 WYETH PHARMACEUTICALS INC. 01/01/1991 01/01/3000 00049 ROERIG 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 07/01/1991 01/01/3000 00053 CSL BEHRING LLC 07/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 07/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. -

2020 Marketing Code of Conduct Compliant Companies
Nevada State Board of Pharmacy 2020 Marketing Code of Conduct Compliant Companies Pursuant to NRS 639.570 Published 11-2-2020 2020 Nevada Marketing Code of Conduct Compliant Companies Manufacturers and Wholesalers Address City ST Zip Submitted under Company Name: Companies, Affiliated Companies or Subsidiaries included on compliance form Abbott Electrophysiology and Heart Failure Abbott Laboratories Abbott Cardiac Rhythm Management Abbott Laboratories Abbott Cardiovascular and Neuromodulation Abbott Laboratories Abbott Diabetes Care Division Abbott Laboratories Abbott Diagnostic Division Abbott Laboratories Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Laboratories Abbott Molecular Division Abbott Laboratories Abbott Neuromodulation Abbott Laboratories Abbott Nutrition Products Division Abbott Laboratories Abbott Point of Care Division Abbott Laboratories Abbott Rapid Diagnostics Abbott Laboratories Abbott Structural Heart Abbott Laboratories Abbott Vascular Division Abbott Laboratories AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 AbbVie, Inc. Acadia Pharmaceuticals, Inc. 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Acadia Pharmaceuticals, Inc Acclarent, Inc. 33 Technology Drive Irvine CA 92618 Acclarent, Inc. Accuri Cyometers, Inc. Becton Dickinson and Company Accutron, Inc Cantel Medical Corp. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Ace Surgical Supply, Inc. Acell, Inc. 6640 Eli Whitney Drive, Suite 200 Columbia MD 21046 Acell, Inc. Acera Surgical, Inc 10880 Baur Blvd St Louis MO 63132 Acera Surgical, Inc Aclaris Therapeutics, Inc. 640 Lee Road, Suite 200 Wayne PA 19087 Aclaris Therapeutics, Inc. Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10502 Acorda Therapeutics, Inc. Johnson & Johnson Pharmaceutical Actelion Pharmaceuticals US, Inc. Affiliates Adamas Pharma, LLC Adamas Pharmaceuticals, Inc.