Tickborne Diseases
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Blood Smear Analysis in Babesiosis, Ehrlichiosis, Relapsing Fever, Malaria, and Chagas Disease
REVIEW STEVE M. BLEVINS, MD RONALD A. GREENFIELD, MD* MICHAEL S. BRONZE, MD CME Assistant Professor of Medicine, Section Professor of Medicine, Section of Infectious Professor of Medicine, Section of Infectious CREDIT of General Internal Medicine, Department Diseases, Department of Medicine, University Diseases, Chair of Department of Medicine, of Medicine, University of Oklahoma of Oklahoma Health Sciences Center and the University of Oklahoma Health Sciences Center Health Sciences Center, Oklahoma City Oklahoma City Veterans Administration and the Oklahoma City Veterans Administration Medical Center Medical Center Blood smear analysis in babesiosis, ehrlichiosis, relapsing fever, malaria, and Chagas disease ■ ABSTRACT LOOD SMEAR ANALYSIS, while commonly B used to evaluate hematologic condi- Blood smear analysis is especially useful for diagnosing tions, is infrequently used to diagnose infec- five infectious diseases: babesiosis, ehrlichiosis, relapsing tious diseases. This is because of the rarity of fever due to Borrelia infection, malaria, and American diseases for which blood smear analysis is indi- trypanosomiasis (Chagas disease). It should be performed cated. Consequently, such testing is often in patients with persistent or recurring fever or in those overlooked when it is diagnostically impor- who have traveled to the developing world or who have tant. a history of tick exposure, especially if accompanied by Nonspecific changes may include mor- hemolytic anemia, thrombocytopenia, or phologic changes in leukocytes and erythro- 1 hepatosplenomegaly. cytes (eg, toxic granulations, macrocytosis). And with certain pathogens, identifying ■ KEY POINTS organisms in a peripheral blood smear allows for a rapid diagnosis. In the United States, malaria and American This paper discusses the epidemiology, trypanosomiasis principally affect travelers from the clinical manifestations, laboratory findings, developing world. -

A Fatal Case of Necrotising Fasciitis of the Eyelid
Br J Ophthalmol: first published as 10.1136/bjo.72.6.428 on 1 June 1988. Downloaded from British Journal of Ophthalmology, 1988, 72, 428-431 A fatal case of necrotising fasciitis of the eyelid R WALTERS From Southampton Eye Hospital, Wilton A venue, Southampton S09 4XW SUMMARY A fatal case of necrotising fasciitis in a 35-year-old man is described and the differential diagnosis and management discussed. Necrotising fasciitis is a potentially fatal skin were taken. The Gram stain revealed Gram-positive infection which is being increasingly recognised as an cocci. He was then treated with intravenous underdiagnosed condition. It requires prompt diag- cefotaxime and gentamicin and topical chlor- nosis, investigation, and treatment. Early surgical amphenicol and gentamicin drops. Because of the debridement is, in combination with suitable intra- poor visual acuity of the right eye it was thought that venous antibiotics, the mainstay of treatment. an orbital cellulitis could not be excluded despite the normal eye movements and absence of proptosis. He Case report was therefore transferred to the General Hospital under the care of an ear, nose, and throat consultant In December 1985 a previously fit 35-year-old factory in order to exclude underlying sinus disease and an manager was referred by his general practitioner to associated abscess. the Casualty Department of the Southampton Eye Skull x-rays (including sinus views) revealed no Hospital with a 12-hour history of increasing redness abnormality and he was therefore continued on his and swelling of his right upper lid. He said that two medical treatment (with the addition of intravenous http://bjo.bmj.com/ days previously he had been poked in the same eye by metronidazole), the presumed diagnosis being his daughter (who had been playing with her guinea- preseptal cellulitis. -

Ehrlichiosis and Anaplasmosis Are Tick-Borne Diseases Caused by Obligate Anaplasmosis: Intracellular Bacteria in the Genera Ehrlichia and Anaplasma
Ehrlichiosis and Importance Ehrlichiosis and anaplasmosis are tick-borne diseases caused by obligate Anaplasmosis: intracellular bacteria in the genera Ehrlichia and Anaplasma. These organisms are widespread in nature; the reservoir hosts include numerous wild animals, as well as Zoonotic Species some domesticated species. For many years, Ehrlichia and Anaplasma species have been known to cause illness in pets and livestock. The consequences of exposure vary Canine Monocytic Ehrlichiosis, from asymptomatic infections to severe, potentially fatal illness. Some organisms Canine Hemorrhagic Fever, have also been recognized as human pathogens since the 1980s and 1990s. Tropical Canine Pancytopenia, Etiology Tracker Dog Disease, Ehrlichiosis and anaplasmosis are caused by members of the genera Ehrlichia Canine Tick Typhus, and Anaplasma, respectively. Both genera contain small, pleomorphic, Gram negative, Nairobi Bleeding Disorder, obligate intracellular organisms, and belong to the family Anaplasmataceae, order Canine Granulocytic Ehrlichiosis, Rickettsiales. They are classified as α-proteobacteria. A number of Ehrlichia and Canine Granulocytic Anaplasmosis, Anaplasma species affect animals. A limited number of these organisms have also Equine Granulocytic Ehrlichiosis, been identified in people. Equine Granulocytic Anaplasmosis, Recent changes in taxonomy can make the nomenclature of the Anaplasmataceae Tick-borne Fever, and their diseases somewhat confusing. At one time, ehrlichiosis was a group of Pasture Fever, diseases caused by organisms that mostly replicated in membrane-bound cytoplasmic Human Monocytic Ehrlichiosis, vacuoles of leukocytes, and belonged to the genus Ehrlichia, tribe Ehrlichieae and Human Granulocytic Anaplasmosis, family Rickettsiaceae. The names of the diseases were often based on the host Human Granulocytic Ehrlichiosis, species, together with type of leukocyte most often infected. -

Tick-Borne Relapsing Fever CLAY ROSCOE, M.D., and TED EPPERLY, M.D., Family Medicine Residency of Idaho, Boise, Idaho
Tick-Borne Relapsing Fever CLAY ROSCOE, M.D., and TED EPPERLY, M.D., Family Medicine Residency of Idaho, Boise, Idaho Tick-borne relapsing fever is characterized by recurring fevers separated by afebrile periods and is accompanied by nonspecific constitutional symptoms. It occurs after a patient has been bitten by a tick infected with a Borrelia spirochete. The diagnosis of tick-borne relapsing fever requires an accurate characterization of the fever and a thorough medical, social, and travel history of the patient. Findings on physical examination are variable; abdominal pain, vomiting, and altered sensorium are the most common symptoms. Laboratory confirmation of tick-borne relapsing fever is made by detection of spirochetes in thin or thick blood smears obtained during a febrile episode. Treatment with a tetracycline or macrolide antibiotic is effective, and antibiotic resistance is rare. Patients treated for tick-borne relapsing fever should be monitored closely for Jarisch- Herxheimer reactions. Fatalities from tick-borne relapsing fever are rare in treated patients, as are subsequent Jarisch-Herxheimer reactions. Persons in endemic regions should avoid rodent- and tick-infested areas and use insect repellents and protective clothing to prevent tick bites. (Am Fam Physician 2005;72:2039-44, 2046. Copyright © 2005 American Academy of Family Physicians.) S Patient information: ick-borne relapsing fever (TBRF) develop with TBRF, with long-term sequelae A handout on tick-borne is transmitted by Ornithodoros that may be permanent. Reviewing a broad relapsing fever, written by 1,3-6 the authors of this article, ticks infected with one of sev- differential diagnosis (Table 1 ) for fever is provided on page 2046. -

Lyme Disease and Tick-Borne Infections User Manual
SCOTTISH MICROBIOLOGY REFERENCE LABORATORY, INVERNESS: LYME DISEASE AND TICK-BORNE INFECTIONS USER MANUAL 1 Amended 23 September 2020 CONTENTS Section Page 1 Introduction 3 2 Contact details and key personnel 3 3 Opening hours 4 4 Service provided 4 4.1 Samples and turnaround times 4 4.2 Laboratory tests 5 4.3 Specialist advice 5 5 Clinical Information 6 6 Referral criteria 6 7 Specimen and request form labelling 7 8 Specimen transportation 8 9 Charges 8 10 Results 8 11 Treatment 8 12 Prevention 8 13 SLDTRL request form 8 (Form MF023) 14 SLDTRL developments 9 15 References 9 16 Laboratory diagnosis of Lyme borreliosis algorithm Appendix 2 Amended 23 September 2020 1.0 Introduction The newly established Scottish Lyme Disease and Tick-borne Infections Reference Laboratory (SLDTRL) is provided by NHS Highland at Raigmore Hospital, Inverness. The aim of SLDTRL is to provide more comprehensive and standardised testing for Lyme disease and other tick-borne infections and to improve the epidemiological data provided to Health Protection Scotland (HPS). Lyme disease is caused by bacteria from the Borrelia burgdorferi sensu lato complex. In the UK the bacteria is transmitted to humans through the bite of infected, hard bodied, Ixodes ricinus ticks. Borrelia miyamotoi disease, which presents as a relapsing fever, can also be transmitted by Ixodes ricinus ticks. It is an emerging disease caused by B. miyamotoi bacteria, which are from the relapsing fever group of borrelia, genetically distinct from those that cause Lyme disease. Human granulocytic anaplasmosis (HGA), also an acute febrile illness transmitted by Ixodid ticks, is an infection caused by the bacterium Anaplasma phagocytophilum. -

Spider Bite Inducing Superficial Lymphangitis: a Case Report
ISSN: 2574-1241 Volume 5- Issue 4: 2018 DOI: 10.26717/BJSTR.2018.12.002232 El Anzi Ouiam. Biomed J Sci & Tech Res Case Report Open Access Spider Bite Inducing Superficial Lymphangitis: A Case Report El Anzi Ouiam*, Meziane Mariam and Hassam Badredine Department of Dermatology-Venereology, Morocco Received: Published: *Corresponding: December author: 04, 2018; : December 17, 2018 El Anzi Ouiam, Department of Dermatology-Venereology, Morocco Abstract Superficial lymphangitis after insect bite is the result of an allergic reaction to the insect antigen. We present the case of a 22-year-old patient with no notable medical history, presented with a pruritic rash on the chest, two days after an insect bite. Unlike bacterial lymphangitis, the site of insectKeywords: bite is not painful but pruritic. Spider Bite; Lymphangitis; Allergic Reaction Introduction lymphadenopathy. Different authors assume that superficial Superficial lymphangitis after insect bite is the result of an lymphangitis after spider sting is the consequence of an allergic allergic reaction to the insect antigen, which is injected into the skin immune reaction due to insect toxins [3]. Unlike bacterial andClinical drained Case by lymphatic vessels [1]. lymphangitis, the site of insect bite is not painful but pruritic. It’s also characterised by the absence of fever and lymph node A 22-year-old female with no notable medical history, presented enlargement and a rapid spontaneous regression [1-3] (Figure 1). with a pruritic rash on the chest. She mentioned intense pruritus and low pain. Two days before she had been bitten on the shoulder by a spider. Physical examination showed a red linear lesion starting from erythematous macule of the shoulder and extending toward the anterior wall of the chest. -

Tick-Borne Diseases in Maine a Physician’S Reference Manual Deer Tick Dog Tick Lonestar Tick (CDC Photo)
tick-borne diseases in Maine A Physician’s Reference Manual Deer Tick Dog Tick Lonestar Tick (CDC PHOTO) Nymph Nymph Nymph Adult Male Adult Male Adult Male Adult Female Adult Female Adult Female images not to scale know your ticks Ticks are generally found in brushy or wooded areas, near the DEER TICK DOG TICK LONESTAR TICK Ixodes scapularis Dermacentor variabilis Amblyomma americanum ground; they cannot jump or fly. Ticks are attracted to a variety (also called blacklegged tick) (also called wood tick) of host factors including body heat and carbon dioxide. They will Diseases Diseases Diseases transfer to a potential host when one brushes directly against Lyme disease, Rocky Mountain spotted Ehrlichiosis anaplasmosis, babesiosis fever and tularemia them and then seek a site for attachment. What bites What bites What bites Nymph and adult females Nymph and adult females Adult females When When When April through September in Anytime temperatures are April through August New England, year-round in above freezing, greatest Southern U.S. Coloring risk is spring through fall Adult females have a dark Coloring Coloring brown body with whitish Adult females have a brown Adult females have a markings on its hood body with a white spot on reddish-brown tear shaped the hood Size: body with dark brown hood Unfed Adults: Size: Size: Watermelon seed Nymphs: Poppy seed Nymphs: Poppy seed Unfed Adults: Sesame seed Unfed Adults: Sesame seed suMMer fever algorithM ALGORITHM FOR DIFFERENTIATING TICK-BORNE DISEASES IN MAINE Patient resides, works, or recreates in an area likely to have ticks and is exhibiting fever, This algorithm is intended for use as a general guide when pursuing a diagnosis. -

Leptospirosis: a Waterborne Zoonotic Disease of Global Importance
August 2006 volume 22 number 08 Leptospirosis: A waterborne zoonotic disease of global importance INTRODUCTION syndrome has two phases: a septicemic and an immune phase (Levett, 2005). Leptospirosis is considered one of the most common zoonotic diseases It is in the immune phase that organ-specific damage and more severe illness globally. In the United States, outbreaks are increasingly being reported is seen. See text box for more information on the two phases. The typical among those participating in recreational water activities (Centers for Disease presenting signs of leptospirosis in humans are fever, headache, chills, con- Control and Prevention [CDC], 1996, 1998, and 2001) and sporadic cases are junctival suffusion, and myalgia (particularly in calf and lumbar areas) often underdiagnosed. With the onset of warm temperatures, increased (Heymann, 2004). Less common signs include a biphasic fever, meningitis, outdoor activities, and travel, Georgia may expect to see more leptospirosis photosensitivity, rash, and hepatic or renal failure. cases. DIAGNOSIS OF LEPTOSPIROSIS Leptospirosis is a zoonosis caused by infection with the bacterium Leptospira Detecting serum antibodies against leptospira interrogans. The disease occurs worldwide, but it is most common in temper- • Microscopic Agglutination Titers (MAT) ate regions in the late summer and early fall and in tropical regions during o Paired serum samples which show a four-fold rise in rainy seasons. It is not surprising that Hawaii has the highest incidence of titer confirm the diagnosis; a single high titer in a per- leptospirosis in the United States (Levett, 2005). The reservoir of pathogenic son clinically suspected to have leptospirosis is highly leptospires is the renal tubules of wild and domestic animals. -
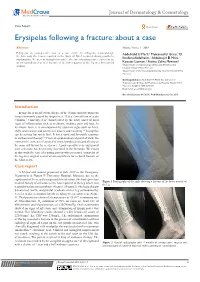
Erysipelas Following a Fracture: About a Case
Journal of Dermatology & Cosmetology Case Report Open Access Erysipelas following a fracture: about a case Abstract Volume 3 Issue 1 - 2019 Erysipelas on postoperative scar is a rare entity. In orthopedic traumatology, Abdelhafid El Marfi,1 Mohamed El Idrissi,1 El we have only the 3cases reported in the work of Dhrif occurred during prosthetic Ibrahimi Abdelhalim,1 Abdelmajid El Mrini,1 implantation. We present through this article, the case of postoperative erysipelas on 2 2 an osteosynthesis scar of a fracture of the lower quarter of the leg in a 58-year-old Kaoutar Laamari, Fatima Zahra Mernissi 1 woman. Department of Traumatology Orthopedy B4, University Hospital Hassan II Fez, Morocco 2Department of Dermatology, University Hospital Hassan II Fez, Morocco Correspondence: Abdelhafid El Marfi, Department of Traumatology Orthopedy B4, University Hospital Hassan II Fez, Morocco, Tel 0021 2678 3398 79, Email Received: January 04, 2018 | Published: January 28, 2019 Introduction Erysipelas is an infectious disease of the dermis and subcutaneous tissue commonly caused by streptococci.1 It is a clinical form of acute cellulitis.2 Clinically, it is characterized by the acute onset of local signs of inflammation such as erythema, oedema, pain and heat. In its classic form, it is accompanied by systemic signs such as fever, chills and malaise and sometimes nausea and vomiting.3,4 Erysipelas can be serious but rarely fatal. It has a rapid and favorable response to antibacterial therapy.5,6 From an epidemiological point of view, we consider the existence of a portal of entry, lymphoedema and obesity as the main risk factors for occurrence. -

Periorbital Necrotising Fasciitis
Eye (1991) 5, 736--740 Periorbital Necrotising Fasciitis GEOFFREY E. ROSE,! DAVID 1. HOWARD,2 MARK R. WATTS! London Summary Three cases of periorbital necrotising fasciitis are described, one occurring in a three-year-old child. The cases in adults required debridement of necrotic tissue, in one of whom there was extensive disease involving the face and orbital fat. ft is probable that the early stages of this condition are under-recognised; the importance of early signs and intensive treatment of this life-threatening disease are illustrated. N ecrotising fasciitis is an ischaemic necrosis of periorbital (and, in one case, facial and intra subcutaneous tissues (fascia), generally due orbital) tissues was required. to an overwhelming infection with �-haemo lytic Streptococcus pyogenes.1,2 The disease Case Reports has a rapid onset, often within a few days of minor breaks in the skin, and spreads exten Case 1 sively and rapidly through subcutaneous fas A 3l-year-old, otherwise healthy, girl was admitted cial planes. to the referring hospital with a 24-hour history of The condition occurs most frequently in the periorbital pain, general malaise, lethargy and a rapidly increasing severe periorbital swelling. For limbs or the abdominal wall, where it has been three days prior to admission, she had been treated given various terms, such as haemolytic strep with oral nystatin for perineal candidiasis and for tococcus gangrene/ gangrenous or necrotis two days had symptoms of an infection of the upper lng erysipelas, suppurative fasciitis and respiratory tract. Her right eyelids were closed by Fournier's gangrene. Periorbital necrotising gross erythematous swelling, but her eye move fasciitis is reported rarely, with a total of only ments were normal and there was no relative affe 16 cases cited in a recent review.1 The low inci rent pupillary defect. -

Ehrlichiosis in Brazil
Review Article Rev. Bras. Parasitol. Vet., Jaboticabal, v. 20, n. 1, p. 1-12, jan.-mar. 2011 ISSN 0103-846X (impresso) / ISSN 1984-2961 (eletrônico) Ehrlichiosis in Brazil Erliquiose no Brasil Rafael Felipe da Costa Vieira1; Alexander Welker Biondo2,3; Ana Marcia Sá Guimarães4; Andrea Pires dos Santos4; Rodrigo Pires dos Santos5; Leonardo Hermes Dutra1; Pedro Paulo Vissotto de Paiva Diniz6; Helio Autran de Morais7; Joanne Belle Messick4; Marcelo Bahia Labruna8; Odilon Vidotto1* 1Departamento de Medicina Veterinária Preventiva, Universidade Estadual de Londrina – UEL 2Departamento de Medicina Veterinária, Universidade Federal do Paraná – UFPR 3Department of Veterinary Pathobiology, University of Illinois 4Department of Veterinary Comparative Pathobiology, Purdue University, Lafayette 5Seção de Doenças Infecciosas, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul – UFRGS 6College of Veterinary Medicine, Western University of Health Sciences 7Department of Clinical Sciences, Oregon State University 8Departamento de Medicina Veterinária Preventiva e Saúde Animal, Universidade de São Paulo – USP Received June 21, 2010 Accepted November 3, 2010 Abstract Ehrlichiosis is a disease caused by rickettsial organisms belonging to the genus Ehrlichia. In Brazil, molecular and serological studies have evaluated the occurrence of Ehrlichia species in dogs, cats, wild animals and humans. Ehrlichia canis is the main species found in dogs in Brazil, although E. ewingii infection has been recently suspected in five dogs. Ehrlichia chaffeensis DNA has been detected and characterized in mash deer, whereas E. muris and E. ruminantium have not yet been identified in Brazil. Canine monocytic ehrlichiosis caused by E. canis appears to be highly endemic in several regions of Brazil, however prevalence data are not available for several regions. -

Antigen Detection Assay for the Diagnosis of Melioidosis
PI: Title: Antigen Detection assay for the Diagnosis of Melioidosis Received: 12/05/2013 FOA: PA10-124 Council: 05/2014 Competition ID: ADOBE-FORMS-B1 FOA Title: NIAID ADVANCED TECHNOLOGY STTR (NIAID-AT-STTR [R41/R42]) 2 R42 AI102482-03 Dual: Accession Number: 3650491 IPF: 3966401 Organization: INBIOS INTERNATIONAL, INC. Former Number: Department: IRG/SRG: ZRG1 IDM-V (12)B AIDS: N Expedited: N Subtotal Direct Costs Animals: N New Investigator: N (excludes consortium F&A) Humans: Y Early Stage Investigator: N Year 3: Clinical Trial: N Year 4: Current HS Code: E4 Year 5: HESC: N Senior/Key Personnel: Organization: Role Category: Always follow your funding opportunity's instructions for application format. Although this application demonstrates good grantsmanship, time has passed since the grantee applied. The sample may not reflect the latest format or rules. NIAID posts new samples periodically: https://www.niaid.nih.gov/grants-contracts/sample-applications The text of the application is copyrighted. You may use it only for nonprofit educational purposes provided the document remains unchanged and the PI, the grantee organization, and NIAID are credited. Note on Section 508 conformance and accessibility: We have reformatted these samples to improve accessibility for people with disabilities and users of assistive technology. If you have trouble accessing the content, please contact the NIAID Office of Knowledge and Educational Resources at [email protected]. Additions for Review Accepted Publication Accepted manuscript news Post-submission supplemental material. Information about manuscript accepted for publication. OMB Number: 4040-0001 Expiration Date: 06/30/2011 APPLICATION FOR FEDERAL ASSISTANCE 3. DATE RECEIVED BY STATE State Application Identifier SF 424 (R&R) 1.