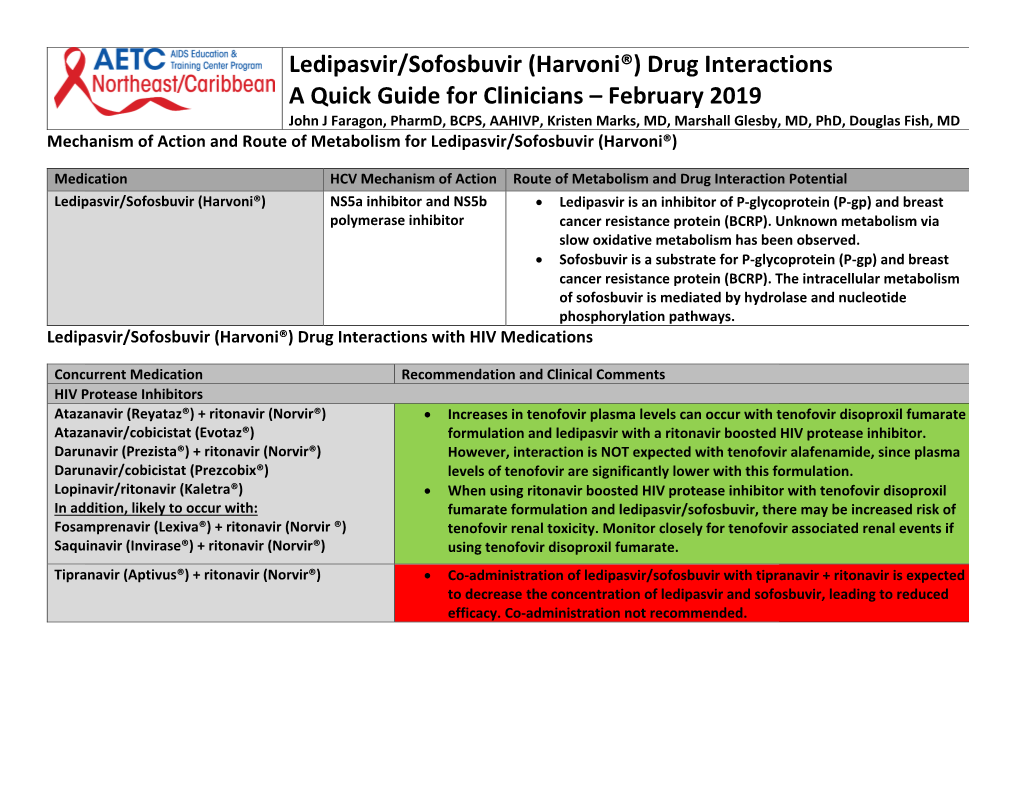
Ledipasvir/Sofosbuvir (Harvoni®) Drug Interactions a Quick Guide For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sofosbuvir for the Treatment of Genotype 1 Hepatitis C in Subjects Aged 65 Years Or Older
AMERICAN ASSOCIATION FOR THE STUDY OFLIVERD I S E ASES HEPATOLOGY, VOL. 63, NO. 4, 2016 Safety and Efficacy of Ledipasvir/ Sofosbuvir for the Treatment of Genotype 1 Hepatitis C in Subjects Aged 65 Years or Older Sammy Saab,1 Sarah H. Park,1 Masashi Mizokami,2 Masao Omata,3 Alessandra Mangia,4 Ed Eggleton,5 Yanni Zhu,5 Steven J. Knox,5 Phil Pang,5 Mani Subramanian,5 Kris Kowdley,6 and Nezam H. Afdhal7 Elderly subjects have been historically underrepresented in clinical trials involving antiviral hepatitis C therapies. The aim of this analysis was to retrospectively evaluate the safety and efficacy of ledipasvir/sofosbuvir (LDV/SOF) by age groups of <65 years versus 65 years among subjects enrolled in phase 3 trials. Four open-label phase 3 clinical trials evaluated the safety and efficacy of LDV/SOF with or without ribavirin (RBV) for the treatment of genotype 1 chronic hepatitis C virus. Sustained virological response at 12 weeks, treatment-emergent adverse events (AEs), and graded laboratory abnor- malities were analyzed according to age group. Of the 2293 subjects enrolled in four phase 3 trials, 264 (12%) were 65 years of age, of whom 24 were aged 75 years. Sustained virological response at 12 weeks was achieved by 97% (1965/ 2029) of subjects aged <65 years and 98% (258/264) of subjects aged 65 years. The most common AEs in both LDV/ SOF groups that occurred in 10% of subjects were headache and fatigue. The rate of study discontinuation due to AEs was similar in the two age cohorts. -

Effectiveness and Safety of Sofosbuvir Plus Ribavirin for the Treatment of HCV Genotype 2 Infection
Hepatology ORIGINAL ARTICLE Effectiveness and safety of sofosbuvir plus ribavirin Gut: first published as 10.1136/gutjnl-2016-311609 on 13 July 2016. Downloaded from for the treatment of HCV genotype 2 infection: results of the real-world, clinical practice HCV-TARGET study Tania M Welzel,1 David R Nelson,2 Giuseppe Morelli,2 Adrian Di Bisceglie,3 Rajender K Reddy,4 Alexander Kuo,5 Joseph K Lim,6 Jama Darling,7 Paul Pockros,8 Joseph S Galati,9 Lynn M Frazier,10 Saleh Alqahtani,11 Mark S Sulkowski,11 Monika Vainorius,7 Lucy Akushevich,7 Michael W Fried,7 Stefan Zeuzem,1 for the HCV-TARGET Study Group ► Additional material is ABSTRACT published online only. To view Objective Due to a high efficacy in clinical trials, Significance of this study please visit the journal online sofosbuvir (SOF) and ribavirin (RBV) for 12 or 16 weeks (http://dx.doi.org/10.1136/ gutjnl-2016-311609). is recommended for treatment of patients with HCV genotype (GT) 2 infection. We investigated safety and What is already known on this subject? For numbered affiliations see effectiveness of these regimens for GT2 in HCV-TARGET ▸ fi end of article. Due to a high ef cacy in clinical trials, the participants. all-oral combination of sofosbuvir (SOF) and Correspondence to Design HCV-TARGET, an international, prospective ribavirin (RBV) for 12 weeks is currently Dr Tania Mara Welzel, JW observational study evaluates clinical practice data on considered standard of care in patients with Goethe University Hospital, novel antiviral therapies at 44 academic and 17 HCV genotype 2 (GT2) infection. -

Sofosbuvir/Ledipasvir for HIV •
Hepatitis C: Drugs and Combinations Melissa Osborn MD Associate Professor MetroHealth Medical Center Case Western Reserve University Cleveland, OH Faculty and Planning Committee Disclosures Please consult your program book. Off-Label Disclosure The following off-label/investigational uses will be discussed in this presentation: • Sofosbuvir/ledipasvir for HIV •. Investigational agents for hepatitis C will be mentioned – Asunaprevir – Daclatasvir – Beclabuvir – Grazoprevir – Elbasvir – GS-5816 Learning Objectives Upon completion of this presentation, learners should be better able to: • apply clinical trial data on new hepatitis C therapies to their patient population. • select which new hepatitis C therapies are appropriate to use with common antiretrovirals. Evolution of interferon-based therapy in HCV-monoinfected genotype 1 patients Sustained Virologic Response 100% 90% 80% 80% 75% 67% 60% 42% 46% 40% 28% 20% 7% 0% Std interferon-alfa IFN + RBV Peg-alfa-2b+RBV Peg-alfa-2a +RBV P/R/Telaprevir P/R/Boceprevir P/R/Simeprevir P/R/Sofosbuvir McHutchison, NEJM 1998; 339: 1485-92 Jacobson, NEJM 2011; 364:2405-16 Fried, NEJM 2002; 347: 975-82 Poordad, NEJM 2011; 364: 1195-206 Manns, Lancet 2001; 358:958-65 Jacobson, AASLD 2013 #1122 Lawitz, NEJM 2013 Evolution of HCV Therapy: Genotype 1 Patients Naïve to Therapy: HIV-HCV coinfection Sustained Virologic Response 80% 75% 74% 67% 61% 60% 46% 42% 40% 28% 29% 20% 17% 7% 7% 0% Std interferon-alfa IFN + RBV Peg-alfa-2b+RBV Peg-alfa-2a +RBV P/R/Telaprevir P/R/Boceprevir Torriani, NEJM, 2004 351:438-50 -

SOVALDI® (Sofosbuvir)
HIGHLIGHTS OF PRESCRIBING INFORMATION --------------------------------CONTRAINDICATIONS----------------------------- These highlights do not include all the information needed to use • When used in combination with peginterferon alfa/ribavirin or SOVALDI safely and effectively. See full prescribing information ribavirin alone, all contraindications to peginterferon alfa and/or for SOVALDI. ribavirin also apply to SOVALDI combination therapy. (4) ® SOVALDI (sofosbuvir) tablets, for oral use -------------------------WARNINGS AND PRECAUTIONS--------------------- Initial U.S. Approval: 2013 • Bradycardia with amiodarone coadministration: Serious symptomatic bradycardia may occur in patients taking ------------------------------RECENT MAJOR CHANGES----------------------- amiodarone and SOVALDI in combination with another direct Indications and Usage (1) 08/2015 acting antiviral (DAA), particularly in patients also receiving beta Dosage and Administration (2.1, 2.2) 08/2015 blockers, or those with underlying cardiac comorbidities and/or Contraindications (4) 08/2015 advanced liver disease. Coadministration of amiodarone with Warnings and Precautions (5.1) 03/2015 SOVALDI in combination with another DAA is not recommended. Warnings and Precautions (5.2, 5.3, 5.4) 08/2015 In patients without alternative, viable treatment options, cardiac -------------------------------INDICATIONS AND USAGE------------------------ monitoring is recommended. (5.1, 6.2,7.1) SOVALDI is a hepatitis C virus (HCV) nucleotide analog NS5B • Use with other drugs containing -
![2015 Hepatitis C Second Generation Antivirals (Harvoni [Ledipasvir/Sofosbuvir], and Viekira Pak [Ombitasvir/Paritaprevir/Ritonavir](https://docslib.b-cdn.net/cover/3879/2015-hepatitis-c-second-generation-antivirals-harvoni-ledipasvir-sofosbuvir-and-viekira-pak-ombitasvir-paritaprevir-ritonavir-293879.webp)
2015 Hepatitis C Second Generation Antivirals (Harvoni [Ledipasvir/Sofosbuvir], and Viekira Pak [Ombitasvir/Paritaprevir/Ritonavir
2015 Hepatitis C Second Generation Antivirals (Harvoni [ledipasvir/sofosbuvir], and Viekira Pak [ombitasvir/paritaprevir/ritonavir + dasabuvir ]) Prior Authorization – Through Preferred Agents Program Summary 2015 Hepatitis C Second Generation Antivirals (Harvoni [ledipasvir/sofosbuvir], and Viekira Pak [ombitasvir/paritaprevir/ritonavir + dasabuvir]) Prior Authorization – Through Preferred Oral Agent(s) The Hepatitis C First Generation, Hepatitis C Second Generation and Sovaldi Prior Authorization Programs must be implemented together. OBJECTIVE The intent of the Hepatitis C second generation antiviral Prior Authorization (PA) program is to appropriately select patients for therapy according to the Food and Drug Administration (FDA) approved product labeling and/or clinical guidelines and/or clinical studies. The PA process will evaluate the use of these agents when there is supporting clinical evidence of Class IIa, Level C or better for their use. Patients requesting Harvoni that are treatment naïve, non-cirrhotic and with an initial viral load < 6 M IU/mL will be approved for 8 weeks assuming all other criteria are met. This criteria does not include the use of Sovaldi (sofosbuvir), and requests for Sovaldi (sofosbuvir) in combination with Olysio (simeprevir) which will not be approved. For the use of Sovaldi (sofosbuvir) see Sovaldi specific criteria. For the use of Olysio in combination with peginterferon and ribavirin, see Hepatitis C First Generation criteria. For hepatocellular carcinoma patients, see Sovaldi (sofosbuvir) specific criteria. TARGET DRUGS Preferred Agent(s) Harvoni® (ledipasvir/sofosbuvir) Nonpreferred Agent(s) Viekira Pak (ombitasvir/paritaprevir/ritonavir + dasabuvir) PRIOR AUTHORIZATION CRITERIA FOR APPROVAL Harvoni (ledipasvir/sofosbuvir) will be approved when the following criteria are met: 1. The patient has a diagnosis of chronic hepatitis C confirmed by serological markers AND 2. -

Hepatitis C Treatment
Hepatitis C Treatment The goal of treatment for hepatitis C virus (HCV) is to cure the virus, which can be done with a combination of drugs. The specific meds used and the duration of treatment depend on a number of factors, including HCV genotype (genetic structure of the virus), viral load, past treatment experience, degree of liver damage, ability to tolerate the prescribed treatment, and whether the person is waiting for a liver transplant or is a transplant recipient. In some cases, HCV treatment may be limited by your health insurance plan or drug formulary. Here’s information about each type, or class, of approved HCV treatment along with drugs in the late stages of development: Multi-Class Combination Drugs Brand Name Generic Name Status Pharmaceutical Company Epclusa* sofosbuvir + velpatasvir Approved Gilead Sciences Harvoni* ledipasvir + sofosbuvir Approved Gilead Sciences Mavyret glecaprevir + pibrentasvir Approved AbbVie Vosevi sofosbuvir/velpatasvir/ Approved Gilead Sciences voxilaprevir Zepatier elbasvir + grazoprevir Approved Merck n/a daclatasvir + asunaprevir + Phase III Bristol-Myers Squibb beclabuvir *generic available What are they? Multi-class combination drugs are a combination of drugs formulated into a single pill or package of pills. For instance, the drug Harvoni combines two drugs, ledipasvir and sofosbuvir. Ledipasvir is an NS5A inhibitor and is only sold as part of Harvoni; sofosbuvir may be prescribed separately under the brand name of Sovaldi. Pegylated Interferon Alfa Brand Name Generic Name Status Pharmaceutical Company Pegasys peginterferon alfa-2a Approved Genentech What are they? Interferon is a protein made by the immune system, named because it interferes with viral reproduction. In addition, interferon signals the immune system to recognize and respond to microorganisms, including viral and bacterial infections. -

Study Protocol and Statistical Analysis Plan
Effects of Ledipasvir/Sofosbuvir on the Pharmacokinetics and Renal Safety of Tenofovir Alafenamide (TAF) NCT03126370 Protocol & Statistical Analysis Plan Version 03.29.2018 COMIRB Protocol COLORADO MULTIPLE INSTITUTIONAL REVIEW BOARD CAMPUS BOX F-490 TELEPHONE: 303-724-1055 Fax: 303-724-0990 Protocol #: 17-0490 Project Title: Effects of ledipasvir/sofosbuvir treatment on the pharmacokinetics and renal safety of tenofovir alafenamide (TAF) Principal Investigator: Jennifer J. Kiser, PharmD Version Number: 3.0 Version Date: 03/29/2018 I. Hypotheses and Specific Aims: Hypothesis: 1. Tenofovir plasma concentrations will be lower, tenofovir diphosphate concentrations in peripheral blood mononuclear cells will be higher, and tenofovir diphosphate concentrations in red blood cells (measured as dried blood spots, DBS) will be lower with tenofovir alafenamide relative to tenofovir disoproxil fumarate. 2. Tenofovir plasma and intracellular tenofovir diphosphate concentrations will increase when tenofovir alafenamide is co-administered with ledipasvir/sofosbuvir relative to TAF alone. 3. Markers of renal function (e.g. CrCl, urinary retinol binding protein/creatinine ratio and urinary beta-2 microglobulin/creatinine ratio) will not change when tenofovir alafenamide is coadministered with ledipasvir/sofosbuvir. Primary Aims: 1. Compare the area under the concentration time curve over the dosing interval (AUC0-24) of tenofovir in plasma when switched from tenofovir disoproxil fumarate (TDF) (Phase 1) to tenofovir alafenamide (TAF 25mg) (Phase 2), and compare the AUC0-24 of tenofovir in plasma given as TDF (Phase 1) to tenofovir in plasma in the form of TAF 25mg concomitantly with ledipasvir (LDV)/sofosbuvir (SOF) (Phase 3) 2. Compare TFV-DP in PBMCs between TDF (Phase 1) and TAF 25mg (Phase 2) and between TDF (Phase 1) and TAF 25mg plus LDV/SOF (Phase 3) Secondary Aims: 1. -

Ledipasvir/Sofosbuvir
Drug and Biologic Coverage Policy Effective Date ............................................ 9/1/2021 Next Review Date… ..................................... 9/1/2022 Coverage Policy Number ............................... IP0186 Ledipasvir/Sofosbuvir Table of Contents Related Coverage Resources Overview .............................................................. 1 Authorized Generics Medical Necessity Criteria ................................... 1 Interferon Therapy Reauthorization Criteria ....................................... 4 Omnibus Codes Authorization Duration ......................................... 4 Conditions Not Covered....................................... 5 Background .......................................................... 6 References .......................................................... 8 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the -

Caracterización Molecular Del Perfil De Resistencias Del Virus De La
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Programa de doctorado en Medicina Departamento de Medicina Facultad de Medicina Universidad Autónoma de Barcelona TESIS DOCTORAL Caracterización molecular del perfil de resistencias del virus de la hepatitis C después del fallo terapéutico a antivirales de acción directa mediante secuenciación masiva Tesis para optar al grado de doctor de Qian Chen Directores de la Tesis Dr. Josep Quer Sivila Dra. Celia Perales Viejo Dr. Josep Gregori i Font Laboratorio de Enfermedades Hepáticas - Hepatitis Víricas Vall d’Hebron Institut de Recerca (VHIR) Barcelona, 2018 ABREVIACIONES Abreviaciones ADN: Ácido desoxirribonucleico AK: Adenosina quinasa ALT: Alanina aminotransferasa ARN: Ácido ribonucleico ASV: Asunaprevir BOC: Boceprevir CCD: Charge Coupled Device CLDN1: Claudina-1 CHC: Carcinoma hepatocelular DAA: Antiviral de acción directa DC-SIGN: Dendritic cell-specific ICAM-3 grabbing non-integrin DCV: Daclatasvir DSV: Dasabuvir -

S Sofosbuvir (Sovaldi )
Sofosbuvir (Sovaldi ™) UTILIZATION MANAGEMENT CRITERIA DRUG CLASS: Nucleotide Analog NS5B Polymerase Inhibitor BRAND (generic) NAME: Sovaldi (sofosbuvir ) 400 mg strength tablet FDA -APPROVED INDICATIONS Sovaldi is a hepatitis C virus (HCV) nucleotide analog NS5B polymerase inhibitor indicated for the treatment of chronic hepatitis C (CHC) infection as a component of a combination antiviral treatment regimen. • Sovaldi efficacy has been established in subjects with HCV genotype 1, 2, 3 or 4 infection, including those with hepatocellular carcinoma meeting Milan criteria (awaiting liver transplantation) and those with HCV/HIV -1 co-infection. COVERAGE AUTHORIZATION CRITE RIA Sofosbuvir (Sovaldi) is cover ed for the following condition and situations: • Diagnosis of chronic hepatitis C (CHC) infection with c onfirmed genotype 1, 2, 3, or 4, OR • CHC infection with hepatocellular carcinoma awaiting liver transplant, AND • Sofosbuvir is prescribed in combination with ribavirin and pegylated interferon alfa for genotypes 1 and 4, OR • Sofosbuvir is prescribed in combination with ribavirin for p atients with genotype 1 who are peginterferon-ineligible* (medical record documentation of ineligibility for peginterferon therapy must be submitted for review), OR • Sofosbuvir is prescribed in combination with simeprevir, with or without ribavirin, for 12 weeks duration of therapy, for patients with genotype 1 that have advanced fi brosis (METAVIR score 2, 3, 4, OR cirrhosis secondary to Hepatitis C ), and are peginterferon - ineligible* (medical record -

HARVONI® (Ledipasvir and Sofosbuvir) Tablets, for Oral Use Weight
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use • Recommended dosage in pediatric patients 3 years and older: HARVONI® safely and effectively. See full prescribing information Recommended dosage of HARVONI in pediatric patients 3 years of for HARVONI. age and older is based on weight. Refer to Table 2 of the full prescribing information for specific dosing guidelines based on body HARVONI® (ledipasvir and sofosbuvir) tablets, for oral use weight. (2.4) HARVONI® (ledipasvir and sofosbuvir) oral pellets • Instructions for Use should be followed for preparation and Initial U.S. Approval: 2014 administration of HARVONI oral pellets. (2.5) WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN • HCV/HIV-1 coinfection: For adult and pediatric patients with PATIENTS COINFECTED WITH HCV AND HBV HCV/HIV-1 coinfection, follow the dosage recommendations in the tables in the full prescribing information. (2.3, 2.4) See full prescribing information for complete boxed warning. • If used in combination with ribavirin, follow the recommendations for ribavirin dosing and dosage modifications. (2.3, 2.4) Hepatitis B virus (HBV) reactivation has been reported, in some • For patients with any degree of renal impairment, including end cases resulting in fulminant hepatitis, hepatic failure, and death. stage renal disease on dialysis, no HARVONI dosage adjustment is (5.1) recommended. (2.6) ------------------------------RECENT MAJOR CHANGES ----------------------- -----------------------DOSAGE FORMS AND STRENGTHS-------------------- Indications and Usage (1) 8/2019 • Tablets: 90 mg of ledipasvir and 400 mg of sofosbuvir; 45 mg of Dosage and Administration ledipasvir and 200 mg of sofosbuvir. (3) Recommended Treatment Regimen and Duration in Patients 3 Years of Age and Older with Genotype 1, 4, 5, • Oral Pellets: 45 mg of ledipasvir and 200 mg of sofosbuvir; 33.75 mg or 6 HCV (2.2) 8/2019 of ledipasvir and 150 mg of sofosbuvir. -

Evaluation of 19 Antiviral Drugs Against SARS-Cov-2 Infection
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.29.067983; this version posted April 29, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. This article is a US Government work. It is not subject to copyright under 17 USC 105 and is also made available for use under a CC0 license. Evaluation of 19 antiviral drugs against SARS-CoV-2 Infection Shufeng Liu1, Christopher Z. Lien1, Prabhuanand Selvaraj1, Tony T. Wang1,* 1Laboratory of Vector-Borne Viral Diseases, Division of Viral Products, Center for Biologics Evaluation, U.S. Food and Drug Administration, Silver Spring, Maryland, United States of America. *e-mail: [email protected] Running Title: Remdesivir inhibits SARS-CoV-2 Abstract The global pandemic of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or 2019- nCoV) has prompted multiple clinical trials to jumpstart search for anti-SARS-CoV-2 therapies from existing drugs, including those with reported in vitro efficacies as well as those ones that are not known to inhibit SARS-CoV-2, such a Ritonavir/lopinavir and Favilavir. Here we report that after screening 19 antiviral drugs that are either in clinical trials or with proposed activity against SARS-CoV-2, remdesivir was the most effective. Chloroquine only effectively protected virus-induced cytopathic effect at around 30 µM with a therapeutic index of 1.5. Our findings also show that velpatasvir, ledipasvir, litonavir, lopinavir, favilavir, sofosbuvir do not have direct antiviral effect. bioRxiv preprint doi: https://doi.org/10.1101/2020.04.29.067983; this version posted April 29, 2020.