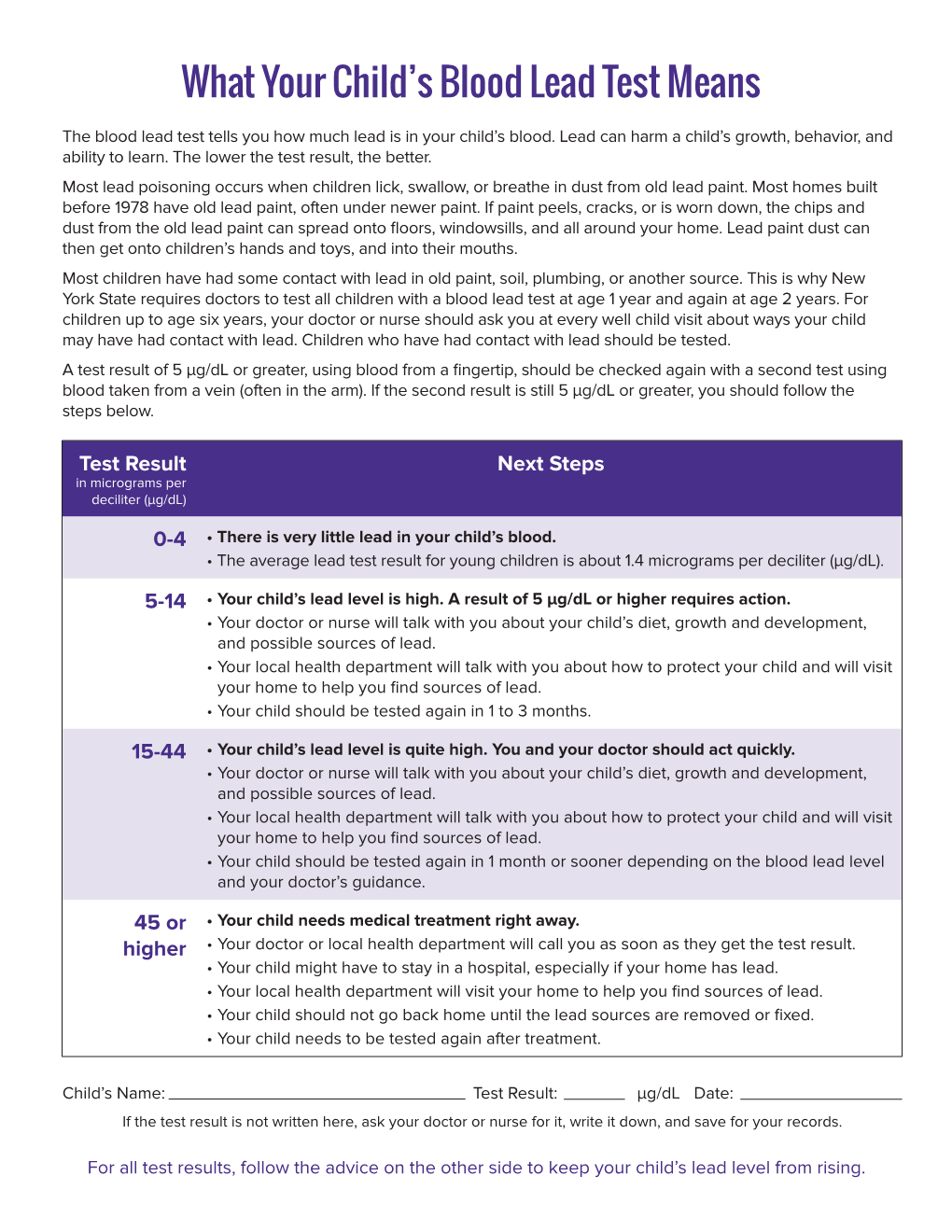
What Your Child's Blood Lead Test Means
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Occurrence of Terrestrial Native Iron in the Earth's Surface
geosciences Article A New Occurrence of Terrestrial Native Iron in the Earth’s Surface: The Ilia Thermogenic Travertine Case, Northwestern Euboea, Greece Christos Kanellopoulos 1,2,* ID , Eugenia Valsami-Jones 3,4, Panagiotis Voudouris 1, Christina Stouraiti 1 ID , Robert Moritz 2, Constantinos Mavrogonatos 1 ID and Panagiotis Mitropoulos 1,† 1 Department of Geology and Geoenvironment, National and Kapodistrian University of Athens, Panepistimioupolis Zografou, 15784 Athens, Greece; [email protected] (P.V.); [email protected] (C.S.); [email protected] (C.M.); [email protected] (P.M.) 2 Section of Earth and Environmental Sciences, University of Geneva, Rue des Maraichers 13, 1205 Geneva, Switzerland; [email protected] 3 School of Geography, Earth & Environmental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK; [email protected] 4 Department of Earth Sciences, Natural History Museum London, Cromwell Road, London SW7 5BD, UK * Correspondence: [email protected] † Professor Panagiotis Mitropoulos has passed away in 2017. Received: 6 April 2018; Accepted: 23 July 2018; Published: 31 July 2018 Abstract: Native iron has been identified in an active thermogenic travertine deposit, located at Ilia area (Euboea Island, Greece). The deposit is forming around a hot spring, which is part of a large active metallogenetic hydrothermal system depositing ore-bearing travertines. The native iron occurs in two shapes: nodules with diameter 0.4 and 0.45 cm, and angular grains with length up to tens of µm. The travertine laminae around the spherical/ovoid nodules grow smoothly, and the angular grains are trapped inside the pores of the travertine. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

Stoichiometry: the Reaction of Iron with Copper(II) Sulfate
CEAC 103 GENERAL CHEMISTRY Experiment 2 Stoichiometry: The Reaction of Iron with Copper(II) Sulfate Purpose: To enhance the understanding of stoichiometry, a reaction between iron and copper (II) sulfate solution will be conducted. This will help you to differentiate limiting and excess reactant in a chemical reaction. Finally the theoretical and percent yield of this reaction will be calculated. Theory Stoichiometry is the measurement of quantitative relationships in chemical formulas and equations. In this experiment stoichiometric principles will be used to obtain the appropriate equation between the reaction of iron metal and copper(II) sulfate solution. After the reaction is taking place, the formation of metallic copper, which is seen precipitating as a finely divided reddish-orange powder will be observed. This reaction is one of the example of single substitution reaction in which one element “displaces” from a compound by another element. The element which has ability of displacing other element from compound is said to be “more active” than the displaced metal. In this experiment, iron is more active than copper. Two distinct forms of iron are present, namely Fe2+ and Fe3+. Stoichiometric principles will be used to determine which reaction is more dominant compared to other one by examining the reaction between iron and copper (II) sulfate solution. If Fe2+ is formed, then equation (1) is dominant, while equation (2) will be selected if Fe3+ is formed. This can be determined 1 according to mole ratio of copper to iron. If the moles of copper is equal to the moles of iron, then equation (1) has taken place. -

Lead in Your Home Portrait Color
Protect Your Family From Lead in Your Home United States Environmental Protection Agency United States Consumer Product Safety Commission United States Department of Housing and Urban Development March 2021 Are You Planning to Buy or Rent a Home Built Before 1978? Did you know that many homes built before 1978 have lead-based paint? Lead from paint, chips, and dust can pose serious health hazards. Read this entire brochure to learn: • How lead gets into the body • How lead afects health • What you can do to protect your family • Where to go for more information Before renting or buying a pre-1978 home or apartment, federal law requires: • Sellers must disclose known information on lead-based paint or lead- based paint hazards before selling a house. • Real estate sales contracts must include a specifc warning statement about lead-based paint. Buyers have up to 10 days to check for lead. • Landlords must disclose known information on lead-based paint or lead-based paint hazards before leases take efect. Leases must include a specifc warning statement about lead-based paint. If undertaking renovations, repairs, or painting (RRP) projects in your pre-1978 home or apartment: • Read EPA’s pamphlet, The Lead-Safe Certifed Guide to Renovate Right, to learn about the lead-safe work practices that contractors are required to follow when working in your home (see page 12). Simple Steps to Protect Your Family from Lead Hazards If you think your home has lead-based paint: • Don’t try to remove lead-based paint yourself. • Always keep painted surfaces in good condition to minimize deterioration. -

Iron –Carbon Phase Diagram
IRON –CARBON PHASE DIAGRAM CB.EN.P2MFG15018 Definition of structures: Various phases that appear on the Iron- Carbon equilibrium phase diagram are as under: • Austenite • Ferrite • Pearlite • Cementite • Martensite • Ledeburite Definition of structures: Austenite is an interstitial solid solution of Carbon dissolved in (F.C.C.) iron. Maximum solubility is 2.0 % C at 1130°C. High formability, most of heat treatments begin with this single phase. It is normally not stable at room temperature. But, under certain conditions it is possible to obtain austenite at room temperature. Austenite Average properties are: Tensile strength = 150,000 psi; Elongation = 10 percent in 2 in.; Hardness = Rockwell C 40, approx; and toughness = high Definition of structures: Ferrite is known as α solid solution. It is an interstitial solid solution of a small amount of carbon dissolved in α (BCC) iron. stable form of iron below 912 deg.C. The maximum solubility is 0.025 % C at 723C and it dissolves only 0.008 % C at room temperature. It is the softest structure that appears on the diagram. Ferrite Average properties are: Tensile strength = 40,000 psi; Elongation = 40 % in 2 in; Hardness > Rockwell C 0 or > Rockwell B 90 Definition of structures: Pearlite is the eutectoid mixture containing 0.80 % C and is formed at 723°C on very slow cooling. It is a very fine platelike or lamellar mixture of ferrite and cementite. The white ferritic background or matrix contains thin plates of cementite (dark). Pearlite Average properties are: Tensile strength = 120,000 psi; Elongation = 20 % in 2 in.; Hardness = Rockwell C20, BHN-300 Definition of structures: Cementite or iron carbide, is very hard, brittle intermetallic compound of iron & carbon, as Fe3C, contains 6.67 % C. -

Of the Periodic Table
of the Periodic Table teacher notes Give your students a visual introduction to the families of the periodic table! This product includes eight mini- posters, one for each of the element families on the main group of the periodic table: Alkali Metals, Alkaline Earth Metals, Boron/Aluminum Group (Icosagens), Carbon Group (Crystallogens), Nitrogen Group (Pnictogens), Oxygen Group (Chalcogens), Halogens, and Noble Gases. The mini-posters give overview information about the family as well as a visual of where on the periodic table the family is located and a diagram of an atom of that family highlighting the number of valence electrons. Also included is the student packet, which is broken into the eight families and asks for specific information that students will find on the mini-posters. The students are also directed to color each family with a specific color on the blank graphic organizer at the end of their packet and they go to the fantastic interactive table at www.periodictable.com to learn even more about the elements in each family. Furthermore, there is a section for students to conduct their own research on the element of hydrogen, which does not belong to a family. When I use this activity, I print two of each mini-poster in color (pages 8 through 15 of this file), laminate them, and lay them on a big table. I have students work in partners to read about each family, one at a time, and complete that section of the student packet (pages 16 through 21 of this file). When they finish, they bring the mini-poster back to the table for another group to use. -

Mercury and Lead Pollution: Still a Critical Issue in Europe
06 December 2007 Mercury and Lead Pollution: still a Critical Issue in Europe Human activities release heavy metals into the atmosphere where they are also transported across national boundaries. This results in air, soil and water pollution through the deposition of heavy metals in environments that are located far away from the actual emission sources. Atmospheric deposition of mercury and lead in particular are calculated to be too high, affecting respectively 51.2% and 7.4% of EU-25 ecosystems respectively in 2000. Heavy metals such as cadmium, lead and mercury can result in serious health risks (e.g. lung damage, kidney diseases, nervous system failures, etc.) and can be harmful to the environment (e.g. soil and water pollution, accumulation in plants). In 1979, the European Union (EU) signed the Long-range Transboundary Air Pollution Convention (LRTAP1), which was the first international legally binding instrument dealing with problems of air pollution on a broad regional basis, including pollution due to heavy metals emissions. In Europe, research programs focusing on heavy metals are already in place, including the EU-funded project ESPREME 2, which aims at developing methods and identifying strategies to support EU environmental policy-making for reducing the emissions and thus the harmful impacts of heavy metals. In the framework of the LRTAP, a methodology 3 was developed to assess the critical loads of heavy metals for both terrestrial and aquatic ecosystems, as well as for human health. The critical load is a measure of how much metal input from anthropogenic sources a system can tolerate. It is the threshold below which significant harmful effects on human health and on the environment do not occur, according to present knowledge. -

Why We Need to Assure Adequacy of Zinc
Aunt Cathy’s Guide to Nutrition: Sanford Nutrition Therapy Dept. 1/15 Thinking about Clinical/Medical Nutrition Issues and Applications of RDAs, RDI, DRIs, ULS, AIs, etc.: Why We Need to Assure Adequacy of Zinc (from the series “How Am I Supposed to Remember All This Stuff?!”) Aunt Cathy Cathy Breedon PhD, RD, CSP, FADA, FAND Clinical and Metabolic Nutrition Specialist / Prenatal/Pediatric Nutrition Specialist Sanford Medical Center, and Clinical Associate Professor UND School of Medicine, Fargo, ND • Zinc is a necessary mineral cofactor needed for over 200 enzymes to work. • Not having enough screws stuff up a LOT. • What kinds of things won’t work well if zinc is inadequate? Read on … 1 Memory Tricks for ZINC Many people remember things best if they also see pictures, so here are some pictures identifying some specific places in the body for which zinc is absolutely essential. Other folks remember things best if they hear them … so if you are one of those people, be sure to read the descriptor for each function of zinc out loud. The Zinc Story: “Zinco” (Zorro’s more irritating cousin) has just marked the places where people need zinc for their bodies to operate. Just picture the fellow on the left to remember that zinc is needed all over the body. It is a co-factor for over 200 enzymes! In other words … I dare you to name a body part that is not dependent on adequacy of zinc! Here are some specific places where he marked things with a Z for Zinc: Zinc is need to make DNA So Zinc is needed And Zinc is needed to to make new cells for babies -

Adverse Health Effects of Heavy Metals in Children
TRAINING FOR HEALTH CARE PROVIDERS [Date …Place …Event …Sponsor …Organizer] ADVERSE HEALTH EFFECTS OF HEAVY METALS IN CHILDREN Children's Health and the Environment WHO Training Package for the Health Sector World Health Organization www.who.int/ceh October 2011 1 <<NOTE TO USER: Please add details of the date, time, place and sponsorship of the meeting for which you are using this presentation in the space indicated.>> <<NOTE TO USER: This is a large set of slides from which the presenter should select the most relevant ones to use in a specific presentation. These slides cover many facets of the problem. Present only those slides that apply most directly to the local situation in the region. Please replace the examples, data, pictures and case studies with ones that are relevant to your situation.>> <<NOTE TO USER: This slide set discusses routes of exposure, adverse health effects and case studies from environmental exposure to heavy metals, other than lead and mercury, please go to the modules on lead and mercury for more information on those. Please refer to other modules (e.g. water, neurodevelopment, biomonitoring, environmental and developmental origins of disease) for complementary information>> Children and heavy metals LEARNING OBJECTIVES To define the spectrum of heavy metals (others than lead and mercury) with adverse effects on human health To describe the epidemiology of adverse effects of heavy metals (Arsenic, Cadmium, Copper and Thallium) in children To describe sources and routes of exposure of children to those heavy metals To understand the mechanism and illustrate the clinical effects of heavy metals’ toxicity To discuss the strategy of prevention of heavy metals’ adverse effects 2 The scope of this module is to provide an overview of the public health impact, adverse health effects, epidemiology, mechanism of action and prevention of heavy metals (other than lead and mercury) toxicity in children. -

Iron in Minnesota's Ground Water
Iron in Minnesota’s Ground Water Environmental Outcomes Division Ground Water May 1999 Monitoring & Assessment What is iron? is soil beneath metal recyclers. However, Program Iron is a chemical element that is widely despite these high concentrations, only distributed in geologic materials. Iron is small quantities of iron are likely to leach slowly released from soil and rocks to to the ground water. ground water. What is considered a safe level of iron The fate of iron in ground water depends in ground water? on the form of iron present. Under The Secondary Maximum Contaminant reducing conditions, ferrous iron (FeII or Level (SMCL) for iron is 0.30 mg/L. Fe+2) occurs. Under oxidizing This standard is not based on human conditions, ferric iron (FeIII or Fe3+) health effects. Iron stains plumbing dominates. Concentrations of ferrous fixtures and clothing. The SMCL is iron in ground or surface water may be based on the concentration of dissolved more than 1 mg/L (part per million). iron in ground water. Ferric iron, although relatively insoluble, forms complexes with other chemicals or How is iron distributed in Minnesota with suspended material. ground water? Iron exceeded the SMCL of 0.30 mg/L What are sources of iron in ground in about 70 percent of the wells sampled water? in the Ground Water Monitoring and Most iron in Minnesota’s ground water Assessment Program (GWMAP) occurs naturally. Iron exists at high statewide baseline network of 954 wells. concentrations in many types of rocks. The concentration of dissolved iron will Concentrations may be as high as 40,000 be smaller since samples were not mg/kg (parts per million) in igneous filtered. -

Three Related Topics on the Periodic Tables of Elements
Three related topics on the periodic tables of elements Yoshiteru Maeno*, Kouichi Hagino, and Takehiko Ishiguro Department of physics, Kyoto University, Kyoto 606-8502, Japan * [email protected] (The Foundations of Chemistry: received 30 May 2020; accepted 31 July 2020) Abstaract: A large variety of periodic tables of the chemical elements have been proposed. It was Mendeleev who proposed a periodic table based on the extensive periodic law and predicted a number of unknown elements at that time. The periodic table currently used worldwide is of a long form pioneered by Werner in 1905. As the first topic, we describe the work of Pfeiffer (1920), who refined Werner’s work and rearranged the rare-earth elements in a separate table below the main table for convenience. Today’s widely used periodic table essentially inherits Pfeiffer’s arrangements. Although long-form tables more precisely represent electron orbitals around a nucleus, they lose some of the features of Mendeleev’s short-form table to express similarities of chemical properties of elements when forming compounds. As the second topic, we compare various three-dimensional helical periodic tables that resolve some of the shortcomings of the long-form periodic tables in this respect. In particular, we explain how the 3D periodic table “Elementouch” (Maeno 2001), which combines the s- and p-blocks into one tube, can recover features of Mendeleev’s periodic law. Finally we introduce a topic on the recently proposed nuclear periodic table based on the proton magic numbers (Hagino and Maeno 2020). Here, the nuclear shell structure leads to a new arrangement of the elements with the proton magic-number nuclei treated like noble-gas atoms. -

Recovery of Silver, Gold, and Lead from a Complex Sulfide Ore Using Ferric Chloride, Thiourea, and Brine Leach Solutions
PLEASE DO NOT REI10VE FROM LIBRARY iRIi9022 i Bureau of M ines Report of Investigations/1986 Recovery of Silver, Gold, and Lead From a Complex Sulfide Ore Using Ferric Chloride, Thiourea, and Brine Leach Solutions By R. G. Sandberg and J. L. Huiatt UNITED STATES DEPARTMENT OF THE INTERIOR Report of Investigations 9022 Recovery of Silver, Gold, and Lead From a Complex Sulfide Ore Using Ferric Chloride, Thiourea, and Brine Leach Solutions By R. G. Sandberg and J. L. Huiatt UNITED STATES DEPARTMENT OF THE INTERIOR Donald Paul Hodel. Secretary BUREAU OF MINES Robert C. Horton. Director Library of Congress Cataloging in Publicatiori Data: Sandberg, R. G. (Richard G.) Recovery of silver, gold, and lead from a complex sulfide ore using ferric chloride, rhiourea, and brine leach solurions. (Repon of invesrigarions / Unired Srares Deparrmenr of rhe Inrerior, Bureau of Mines; 9022) Bibliography: p. 14. Supr. of Docs. no.: I 28.23: 9022. 1. Silver-Merallurgy. 2. Gold-Merallurgy. 3. Lead-Merallurgy. 4. Leaching. 5. Ferric chloride. 6. Salr. I. Huiarl, J. L. II. Tide. III. Series: Repon of invesrigarions (Unired Srares. Bureau of Mines) ; 9022. TN23.U43 [TN770] 6228 (669'.231 1\6-600056 CONTENTS Abstract............................................................................................................... 1 Introduction. .......................................................................................................... 2 Materials.. .. .. .. .. .. .. .. .. .. .. .... .. .. ...... .. .. .. .. • .. .. .. .• .. ... .. .. .. .. .. .. .