Mathematics at the Frontier of Developmental Biology
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Retail Shops, Restaurants and Services
SHOPPING CENTRE KING GEORGE BOULEVARD & 102 AVENUE SURREY, BRITISH COLUMBIA A true downtown experience! Central City offers retail shops and services, “AAA” office space and an internationally recognized university campus all in one location. RETAIL SHOPS, RESTAURANTS AND SERVICES Long known as the hub for retail in Downtown Surrey, Central City promotes a commercial environment by combining local, regional and national Tenants with a 560,000 SF, triple “A” (AAA) office tower and post secondary campuses. The shopping centre spans two levels anchored by Walmart, T&T Supermarket in addition to: The Brick Winners Buck or Two Best Buy Bluenotes XIMI Vogue Urban Planet / Warehouse One Ardene Urban Kids Rogers Wireless / Canadian Passport Bentley WOW / Fido Office Dollarama Telus Mobility / Koodo Prospera Credit Union Central City The Shoe Company Bell Mobile Liquor Store Shoppers Drug Mart Pearle Vision WIRELESSWAVE Club 16 Trevor Linden Image Optometry Freedom Mobile Fitness Foot Locker Alive Health Centre ProfessioNail With excellent food service tenants including: Boston Pizza Coco Fresh Tea Neptune Seafood Restaurant A&W Tim Hortons Booster Juice Starbucks Manchu Wok Blenz Coffee Opa! Souvlaki Burger King Freshslice Pizza KFC Quesada Burritos & Tacos Subway Thai Express Dairy Queen Steve’s Poke Bar Orange Julius Ricky’s All Day Grill Central City is home to the Fraser Valley campus of Simon Fraser University and Stenberg College. Located within walking distance is Kwantlen Polytechnic University (KPU) Civic Plaza campus, Douglas College, West Coast College of Healthcare and Sprott-Shaw College. CENTRAL CITY KEY FACTS 560,000 SF 3,800 Stalls Shopping Centre GLA Onsite Parking 140 $1,600 PSF Retail Stores (approximately) Food Court Sales (average 2019) 365,000 SF 1,000 + Simon Fraser University Daily Visitors to Canadian Surrey Campus Passport Office LOCATION AND ACCESSIBILITY Central City is at the epicentre of Downtown Surrey, fronting King George Boulevard between 100 Avenue and 102 Avenue. -

ORDER FORM Name of Student: ______Division/Grade: ______Total # of Shoppiong Cards ______
ORDER FORM Name of Student: _______________________ Division/Grade: _______________ total # of Shoppiong Cards __________ Name of puchuser: _______________________ Email: _____________________________ Department Stores: Grocery Stores: (DB) Giant Tiger 3.0% x $25.00 (DB) MarketPlace IGA 5.0% x $50.00 $ __________ x $100.00 $ __________ **Reloadable** (DB) Hudson's Bay (Hbc, 3.0% x $25.00 (DB) Loblaws(Shop Easy, 3.0% x $25.00 Home Outfitters) x $50.00 Extra Foods, Superstore,Lucky x $50.00 x $100.00 $ __________ Dollar,No Frills,Canadian Wholesale x $100.00 (DB) London Drugs 2.0% x $25.00 City Market) x $250.00 $ __________ x $50.00 (DB) Safeway, Sobeys and 4.0% x $25.00 x $100.00 $ __________ Thrifty Foods x $50.00 (DB) Walmart 2.0% x $25.00 x $100.00 x $50.00 x $250.00 $ __________ x $100.00 (DB) Save-On-Foods, Urban Fare 4.0% x $25.00 x $250.00 $ __________ Price Smart Foods, x $50.00 Overwaitea Foods x $100.00 Fuel: x $250.00 $ __________ (C) Chevron 2.0% x $25.00 $ __________ (DB) Stong's 5.0% x $50.00 (DB) Esso 2.0% x $25.00 x $100.00 x $50.00 x $500.00 $ __________ x $100.00 $ __________ (DB) T&T Supermarkets 2.0% x $25.00 (DB) Petro-Canada 2.0% x $25.00 (Osaka) x $50.00 $ __________ x $50.00 x $100.00 $ __________ Specialty Stores: (DB) Shell 2.0% x $25.00 (DB) Amazon.ca 2.0% x $25.00 x $50.00 x $50.00 x $100.00 $ __________ x $100.00 $ __________ (DB) American Eagle 5.0% x $25.00 $ __________ Home Improvement: (DB) Bath and Body Works 5.0% x $25.00 $ __________ (DB) Home Depot 2.0% x $25.00 (DB) Best Buy 2.0% x $25.00 x $50.00 -

COLLINGWOOD MIXED-USE RENTAL DEVELOPMENT SITE $167/SF Buildable
FOR SALE COLLINGWOOD MIXED-USE RENTAL DEVELOPMENT SITE $167/SF buildable 3070 Kingsway, Vancouver Plans in place—zoning enactment May 2019 KINGSWAY RUPERT ST JOYCE- COLLINGWOOD Approved 42-unit six-storey development JOYCE ST Mark Goodman Cynthia Jagger Goodman Commercial Inc. Greater Vancouver’s authority on selling Personal Real Estate Corporation Personal Real Estate Corporation 560–2608 Granville St apartment buildings and development sites Direct 604 714 4790 Direct 604 912 9018 Vancouver, BC V6H 3V3 www.goodmanreport.com [email protected] [email protected] 11X17 Public VanMap MIXED-USE MULTI-FAMILY RENTAL DEVELOPMENT SITE Site Plan Address 3070 Kingsway, Vancouver PIDs 011-986-565, 011-986-549 KINGSWAY Legal Lot 1 & 2, Except Part In Reference Plan 2424, Block 16 District Lot 37 Plan 3952 Zoning CD-1 (Comprehensive Development) RUPERT ST enactment set for May 28, 2019 Lot size 10,676 SF Development Six-storey mixed-use building with 41 KERR ST potential rental suites with at grade commercial 10,676 SF SCHOOL AVE PROPOSED SUITE MIX *2019 Type Units Avg. size (SF) Avg. rent ($) Studio 12 466 SF $1,688 OPPORTUNITY 1 bedroom 15 512 SF $1,964 Rare opportunity to acquire a C-2 zoned lot improved with an Rezoning application 2 bedroom 10 743 SF $2,581 existing one-storey commercial building in Vancouver’s 2017 Submitted Nov, 2017 2 bedroom TH 1 822 SF $2,581 Collingwood neighbourhood. Rezoning has been approved 2018 Community open house Feb 5, 2018 3 bedroom TH 3 1,292 SF $3,400 to CD-1 for a six-storey mixed-use building with a three-storey Urban Design Panel Feb 21, 2018 townhouse development at the lane. -

Schoolbucks Order Deadline: Thursday, Feb
Order deadline: Thursday, Jan. 21 → Pick-up date: Thursday, Jan.28 SchoolBucks Order deadline: Thursday, Feb. 4 → Pick-up date: Thursday, Feb. 10 Order deadline: Thursday, Feb. 18 → Pick-up date: Thursday, Feb. 25 S T . T H O M A S A Q U I N A S Order deadline: Thursday, Mar. 4 → Pick-up date: Thursday, Mar. 11 Order deadline: Thursday, Apr. 8 → Pick-up date: Thursday, Apr. 15 Order deadline: Thursday, Apr. 22 → Pick-up date: Thursday, Apr.29 Order deadline: Thursday, May 6 → Pick-up date: Thursday, May 13 Order deadline: Thursday, May 20 → Pick-up date: Thursday, May27 Order deadline: Thursday, June 3 → Pick-up date: Thursday, June 10 Name (please print): Name of student(s): Grade(s): Email Address: Cell Number: I authorize the student(s) listed above to pick-up my SchoolBucks order: Parent’s signature: Date: To complete this form: 1. On the reverse side, choose the retailers and write the number of certificates/gift cards you wish to purchase. 2. Total the amounts. 3. Fill in Method of Payment and Total Payment below. 4. Submit your order and payment to the school office by the ORDER Deadline date (above) by 3:30pm. 5. Please make cheques payable to STA. METHOD OF PAYMENT: Cheque Amount $ Cash Amount $ Cheque Number TOTAL ORDER $ SCHOOLBUCKS PICK-UP: • Please pick-up your SchoolBucks at the school on the specified Pick-Up Date (above) after 3:00 pm. • If you are unable to pick-up at that time, you may pick- up your order at the school office anytime during school office hours (8:00 am - 4:00 pm). -

Christmas 2020 Supporter Order Form
CHRISTMAS 2020 SUPPORTER ORDER FORM NAME OF SUPPORTER: _______________________ PHONE NUMBER: ___________________________ DATE: _____________________ ADDRESS: _______________________________ POSTAL CODE: ___________________________ EMAIL: _____________________ NAME OF PARENT: _______________________ ______________________ GRADE/CLASS: ___________________________ MISC: _____________________ o This is a one time order o This is my new standing order. MERCHANT(FEE) CERTIFICATES TOTAL MERCHANT(FEE) CERTIFICATES TOTAL Department Stores: Grocery Stores: (DB) Giant Tiger 3.0% x $25.00 (DB) Fresh St. Market 5.0% x $50.00 $ __________ x $100.00 $ __________ (DB) MarketPlace IGA 5.0% x $50.00 $ __________ (DB) Hudson's Bay (Hbc, 3.0% x $25.00 **Fresh St M and IGA Reloadable** Home Outfitters) x $50.00 (DB) Loblaws(President's Choice, 3.0% x $25.00 x $100.00 $ __________ Extra Foods, Superstore,Lucky x $50.00 (DB) London Drugs 2.0% x $25.00 Dollar,No Frills,Canadian Wholesale, x $100.00 x $50.00 City Market, Shop Easy) x $250.00 $ __________ x $100.00 $ __________ (DB) Safeway 4.0% x $25.00 (DB) Walmart 3.0% x $25.00 x $50.00 x $50.00 x $100.00 x $100.00 x $250.00 $ __________ x $250.00 $ __________ (DB) Save-On-Foods, Urban Fare 5.0% x $25.00 Price Smart Foods, x $50.00 Fuel: Overwaitea Foods x $100.00 (C) Chevron 2.0% x $25.00 x $250.00 $ __________ x $50.00 (DB) Stong's 5.0% x $50.00 x $100.00 $ __________ x $100.00 (DB) Esso 2.0% x $25.00 x $500.00 $ __________ x $50.00 (DB) T&T Supermarkets 2.0% x $25.00 x $100.00 $ __________ (Osaka) x $50.00 -

2017 BC Championships
2017 BC Championships Prince George Minor Hockey Association March 18th – 23rd “Lead, Develop and Promote Positive Lifelong Hockey Experiences” 1 | P a g e Welcome to the 2017 Midget Tier 1 BC Championships! Your host for the week is the Prince George Midget Tier 1 Cougars. We welcome your team, players and families to Prince George and look forward to hosting you. In this Welcome Package, you will find information about the following: Our Community Championship Contacts General Championship Information Accommodations Kin Centre Arenas Restaurants Points of Interest Skate Sharpening & Sports Stores Transportation Medical Information & Emergency Planning If you have any questions, please contact Liza Arnold [email protected] (250-565-4698). Feel free to call or text during the Championships if you have any questions or concerns. Good luck to all teams and players, PG Midget Tier 1 Cougars 2 | P a g e CONTACT INFORMATION: Prince George Minor Hockey Association - (250) 563-0303 o Street Address . 2187 Ospika Blvd . Prince George, BC . V2M 1W1 o Mailing Address . P.O. Box 2242 . Prince George, BC . V2N 2J8 GENERAL CHAMPIONSHIP INFORMATION: Host Association Championship Contact o Liza Arnold (250) 565-4698 or [email protected] BC Hockey Representative – TBA Schedule of Events – o Times and Locations to be confirmed . March 18th Travel Day . March 19th Banquet and Guest Speaker Coaches Meeting (prior to banquet) . March 20 – 23rd Championship Games 3 | P a g e COMMUNITY INFORMATION: http://www.hellobc.com/prince-george.aspx Prince George is the largest city in Northern British Columbia, and it is located centrally in the provinces. -

Bc Supporter Order Form
BC SUPPORTER ORDER FORM NAME OF SUPPORTER: _______________________ PHONE NUMBER: ___________________________ DATE: _____________________ ADDRESS: _______________________________ POSTAL CODE: ___________________________ EMAIL: _____________________ NAME OF PARENT: _______________________ ______________________ GRADE/CLASS: ___________________________ NOTES: _____________________ o This is a one time order o This is my new standing order. MERCHANT(FEE) CERTIFICATES TOTAL MERCHANT(FEE) CERTIFICATES TOTAL Department Stores: Grocery Stores: (DB) Giant Tiger 3.0% x $25.00 (DB) MarketPlace IGA 5.0% x $50.00 $ __________ x $100.00 $ __________ **Reloadable** (DB) Hudson's Bay (Hbc, 3.0% x $25.00 (DB) Fresh St. Market 5.0% x $50.00 $ __________ Home Outfitters) x $50.00 **Reloadable** x $100.00 $ __________ (DB) Loblaws (Superstore, Extra 3.0% x $25.00 (DB) London Drugs 2.0% x $25.00 Foods, City Market, No Frills, x $50.00 x $50.00 Canadian Wholesale) x $100.00 x $100.00 $ __________ x $250.00 $ __________ (DB) Walmart 3.0% x $25.00 (DB) Safeway, Sobeys and 4.0% x $25.00 x $50.00 Thrifty Foods x $50.00 x $100.00 x $100.00 x $250.00 $ __________ x $250.00 $ __________ (DB) Save-On-Foods, Urban Fare 4.0% x $25.00 Fuel: Price Smart Foods, x $50.00 (DB) Chevron 2.0% x $25.00 $ Overwaitea Foods x $100.00 x $50.00 x $250.00 $ __________ x $100.00 $ __________ (DB) Stong's 4.0% x $50.00 (DB) Esso 2.0% x $25.00 x $100.00 x $50.00 x $500.00 $ __________ x $100.00 $ __________ (DB) T&T Supermarkets 2.0% x $25.00 (DB) Petro-Canada 2.0% x $25.00 (Osaka) x -

White Rock Business Needs Assessment
White Rock Business Needs Assessment Draft Report for Discussion Purposes August 2011 Prepared for: The City of White Rock and the White Rock Business Improvement Association By: Coriolis Consulting Corp. WHITE ROCK BUSINESS NEEDS ASSESSMENT Table of Contents 1.0 INTRODUCTION ..................................................................................................... 1 1.1 Background, Objectives, and Scope .................................................................................. 1 1.2 Approach ............................................................................................................................... 2 2.0 TRADE AREA DESCRIPTION ................................................................................ 3 2.1 Location in a Regional Context ........................................................................................... 3 2.2 Existing Pattern of Commercial Development in the Trade Area .................................... 4 3.0 COMMERCIAL SUB-AREAS IN WHITE ROCK ..................................................... 6 3.1 Location of Commercial Sub-Areas in White Rock .......................................................... 6 3.2 Profile of the Upper Town Centre and Lower Town Centre ............................................. 7 3.2.1 Retail Form and Character .................................................................................... 7 3.2.2 Zoning and OCP Designations .............................................................................. 7 3.2.3 Lease Rates and -

Exhibitor List
EXHIBITOR LIST 1-9 BizComm Inc., Brokerage Copper Branch F www.bizcomm.ca www.copperbranch.ca 180 Smoke Vape Store .................................................... 508 .................................................... 432 Federal Wireless www.180smoke.ca Communications Inc .................................................... 202 Bodhi Bar - Organic Juice and Crackboom www.federalwc.com Smoothie Bar www.crackboom.ca .................................................... 150 241 Pizza www.bodhibar.ca ............................................ 436, 438 www.241pizza.com .................................................... 133 FlagFranchise .....................................523, 525, 527 Cultures www.flagfranchise.com A Boustan www.cultures-restaurants.com ............................................ 441, 443 www.boustan.ca ...................101,103,105,107,109,111 AGTA Home Health Care ............................................. 402,404 Foodtastic Curb-Ease www.foodtastic.ca www.agtafranchises.com www.curb-easefranchise.com ............................................ 126, 128 B-Protek ............................................. 305,307 www.bprotek.com .................................................... 701 Franchise Marketing Aisle 24 .................................................... 236 Custodia Senior Support www.aisle24.ca Systems BrandLoyal www.custodia.com .................................................... 428 ............................................ 820, 822 www.fmsfranchise.com www.brandloyal.io ................................................... -
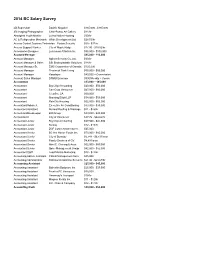
2014 BC Salary Survey Part 1
2014 BC Salary Survey 2D Supervisor Double Negative $165,000 - $185,000 3D Imaging Photographer Chali-Rosso Art Gallery $21/hr Aboriginal Youth Mentor Lu'ma Native Housing $30/hr AC & Refrigeration Mechanic White Development Ltd. $28.50/hr Access Control Systems Technician Fusion Security $30 - $37/hr Access Support Worker City of Maple Ridge $17.80 - $18.82/hr Accessories Designer Lululemon Athletica Inc. $85,000 - $100,000 Account Manager $45,000 - 110,000 Account Manager Agilent Security Co. Ltd. $30/hr Account Manager & Sales BSI Biodegradable Solutions $14/hr Account Manager Sr, EMC Corporation of Canada $125,000 Account Manager Provincial Tank Lining $30,000 - $50,000 Account Manager Rasplayer $40,000 + Commission Account Sales Manager DRMG Envelope $500/Weekly + Comm Accountant $33,000 – 185,000 Accountant Big Chip Accounting $40,000 - $50,000 Accountant CareCorp Vancouver $47,000 - $60,000 Accountant Clearline CA $60,000 Accountant Manning Elliott LLP $38,000 - $58,000 Accountant RainCity Housing $52,000 - $55,000 Accountant/Admin Jr. Executive Air Conditioning $33,000 - $35,000 Accountant Assistant Burrard Roofing & Drainage $21 - $26/hr Accountant/Bookkeeper EM Group $33,000 - $43,000 Accountant II City of Vancouver $37.76 - $44.62/hr Accountant Junior Big Chip Accounting $37,500 - $42,500 Accountant Junior Sonray $12 - $15/hr Accountant Junior ZGF Cotter Architects Inc. $45,000 Accountant Senior BC Hot House Foods Inc. $70,000 - $85,000 Accountant Senior City of Burnaby $5,747 - $6,791/mon Accountant Senior Family Services of GV $4,491/mon Accountant Senior Mimi C. Cheung & Asso. $52,000 - $60,000 Accountant Senior Spice Management Group $42,000 - $52,000 Accountant Staff Lead Mantra Marketing $18 - $22/hr Accounting Admin. -

18Nov21 Op Finance Committee Agenda
NOTICE OF MEETING Vancouver School Board Secretary-Treasurer’s Office Finance Committee (Pending Board Approval) November 16, 2018 Oliver Hanson Lois Chan-Pedley Fraser Ballantyne Estrellita Gonzalez Suzanne Hoffman, Superintendent of Schools J. David Green, Secretary-Treasurer Notice of Meeting A meeting of the Finance Committee will be held in Committee Room # 180 of the Education Centre, 1580 West Broadway, Vancouver, British Columbia, on Wednesday, November 21, 2018 at 5:30 pm. Trustees: Carmen Cho Jennifer Reddy Janet Fraser Allan Wong Barb Parrott Student Trustee: Hazel Pangilinan District Management Staff: Carmen Batista Lisa Landry Aaron Davis Jody Langlois John Dawson Patricia MacNeil Pedro da Silva Jim Meschino Mette Hamaguchi David Nelson Joann Horsley-Holwill Lorelei Russell Magdalena Kassis Rob Schindel Michele Kelly Shehzad Somji Adrian Keough Richard Zerbe Brian Kuhn Reps: Terry Stanway, VSTA Alt. Allison Jambor, VESTA Leslie Roosa, VESTA Doug Matear, VASSA David Bach, VASSA Harjinder Sandhu/Doug Roch, VEPVPA David Murphy, VEPVPA May Ke, DPAC Amanda Hillis, DPAC Peter Powell, PASA Warren Williams, CUPE 15 Thomas Leung, CUPE 15 Charleen Ann Derzak, CUPE 407 Brent Boyd, CUPE 407 Stephen Kelly, Trades Raymond Szczecinski,Trades Harjit Khangura, IUOE Tim DeVivo, IUOE Jonathan Zhu, VDSC Others: Secretary-Treasurer’s Office Ed. Centre Engineers District Parents Rentals Chris Allen Kathie Currie, CUPE 15 Lynda Bonvillain Communications Will Hsu VANCOUVER SCHOOL BOARD COMMITTEE MEETING FINANCE COMMITTEE Wednesday, November 21, 2018 at 5:30 pm Room 180, VSB Education Centre AGENDA The meeting is being held on the traditional unceded territory of the Musqueam, Squamish and Tsleil- Waututh Coast Salish peoples. Meeting Decorum: The Board has a strong commitment to ethical conduct. -

BC Supporter Order Form Mar 2021.Xls
SUPPORTER ORDER FORM NAME OF SUPPORTER: _______________________ PHONE NUMBER: ___________________________ DATE: _____________________ ADDRESS: _______________________________ POSTAL CODE: ___________________________ EMAIL: _____________________ NAME OF PARENT: _______________________ ______________________ GRADE/CLASS: ___________________________ NOTES: _____________________ o This is a one time order o This is my new standing order. MERCHANT & REBATE CERTIFICATES TOTAL MERCHANT & REBATE CERTIFICATES TOTAL Department Stores: Grocery Stores: (DB) Giant Tiger 3.0% x $25.00 (DB) MarketPlace IGA 5.0% x $50.00 $ __________ x $100.00 $ __________ **Reloadable** (DB) HUdson's Bay (Hbc, 3.0% x $25.00 (DB) Fresh St. Market 5.0% x $50.00 $ __________ Home OUtfitters) x $50.00 **Reloadable** x $100.00 $ __________ (DB) Loblaws (SUperstore, Extra 3.0% x $25.00 (DB) London DrUgs 2.0% x $25.00 Foods, City Market, No Frills, x $50.00 x $50.00 Canadian Wholesale) x $100.00 x $100.00 $ __________ x $250.00 $ __________ (DB) Walmart 3.0% x $25.00 (DB) Safeway, Sobeys and 4.0% x $25.00 x $50.00 Thrifty Foods x $50.00 x $100.00 x $100.00 x $250.00 $ __________ x $250.00 $ __________ (DB) Save-On-Foods, Urban Fare 4.0% x $25.00 Fuel: Price Smart Foods, x $50.00 (DB) Chevron 2.0% x $25.00 $ Overwaitea Foods x $100.00 x $50.00 x $250.00 $ __________ x $100.00 $ __________ (DB) Stong's 4.0% x $50.00 (DB) Esso 2.0% x $25.00 x $100.00 x $50.00 x $500.00 $ __________ x $100.00 $ __________ (DB) T&T SUpermarkets 2.0% x $25.00 (DB) Petro-Canada 2.0% x $25.00 (Osaka)