(Corvus Corax) and Its Comparative Evolution Jacobs, Ivo; Kabadayi, C
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Zoologische Verhandelingen
Systematic notes on Asian birds. 45. Types of the Corvidae E.C. Dickinson, R.W.R.J. Dekker, S. Eck & S. Somadikarta With contributions by M. Kalyakin, V. Loskot, H. Morioka, C. Violani, C. Voisin & J-F. Voisin Dickinson, E.C., R.W.R.J. Dekker, S. Eck & S. Somadikarta. Systematic notes on Asian birds. 45. Types of the Corvidae. Zool. Verh. Leiden 350, 26.xi.2004: 111-148.— ISSN 0024-1652/ISBN 90-73239-95-8. Edward C. Dickinson, c/o The Trust for Oriental Ornithology, Flat 3, Bolsover Court, 19 Bolsover Road, Eastbourne, East Sussex, BN20 7JG, U.K. (e-mail: [email protected]). René W.R.J. Dekker, National Museum of Natural History, P.O. Box 9517, 2300 RA Leiden, The Netherlands (e-mail: [email protected]). Siegfried Eck, Staatliche Naturhistorische Sammlungen Dresden, Museum für Tierkunde, A.B. Meyer Bau, Königsbrücker Landstrasse 159, D-01109 Dresden, Germany (e-mail: [email protected]. sachsen.de). Soekarja Somadikarta, Dept. of Biology, Faculty of Science and Mathematics, University of Indonesia, Depok Campus, Depok 16424, Indonesia (e-mail: [email protected]). Mikhail V. Kalyakin, Zoological Museum, Moscow State University, Bol’shaya Nikitskaya Str. 6, Moscow, 103009, Russia (e-mail: [email protected]). Vladimir M. Loskot, Department of Ornithology, Zoological Institute, Russian Academy of Science, St. Petersburg, 199034 Russia (e-mail: [email protected]). Hiroyuki Morioka, Curator Emeritus, National Science Museum, Hyakunin-cho 3-23-1, Shinjuku-ku, Tokyo 100, Japan. Carlo Violani, Department of Biology, University of Pavia, Piazza Botta 9, 27100 Pavia, Italy (e-mail: [email protected]). -

Habitat Use and Population Dynamics of the Azure-Winged Magpie, Cyanopica Cyanus, and Their Response to Fire in Northern Mongolia
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Habitat Use and Population Dynamics of the Azure-Winged Magpie, Cyanopica cyanus, and their Response to Fire in Northern Mongolia A thesis submitted in partial fulfilment of the requirements for the Degree of Master of International Nature Conservation (M.I.N.C) at Lincoln University by Haojin Tan Lincoln University, New Zealand/ Georg-August University, Germany 2011 The Azure-winged Magpie Cyanopica cyanus in Khonin Nuga, Northern Mongolia. Photo by Kerry- Jayne Wilson (2010) ii Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Master of International Nature Conservation Abstract Habitat Use and Population Dynamics of the Azure-winged Magpie, Cyanopica cyanus, and their Response to Fire in Northern Mongolia by Haojin Tan Fires are natural distubances in many ecosystems, but humans have altered fire regimes throughout the world. The effect of fire on organisms, particularly birds, depend on the extent and regime of the fire and the species’ ecology. The Azure-winged Magpie, Cyanopica cyanus is a cooperative breeder, and occurs in a disjunct distribution across much of Asia and in Iberia (Portugal and Spain). -

Evolution of Corvids and Their Presence in the Neogene and the Quaternary in the Carpathian Basin
Ornis Hungarica 2020. 28(1): 121–168. DOI: 10.2478/orhu-2020-0009 Evolution of Corvids and their Presence in the Neogene and the Quaternary in the Carpathian Basin Jenő (Eugen) KESSLER Received: September 09, 2019 – Revised: February 12, 2020 – Accepted: February 18, 2020 Kessler, J. (E.) 2020. Evolution of Corvids and their Presence in the Neogene and the Quater- nary in the Carpathian Basin. – Ornis Hungarica 28(1): 121–168. DOI: 10.2478/orhu-2020-0009 Abstract: Corvids are the largest songbirds in Europe. They are known in the avian fauna of Europe from the Miocene, the beginning of the Neogene, and are currently represented by 11 species. Due to their size, they occur more frequently among fossilized material than other types of songbirds, and thus have been examined to the largest extent. In the current article, we present their known evolution and their fossilized taxa in Europe and examine the osteology of extant species. Keywords: Corvidae, Neogene, Quaternary, Europe, Carpathian Basin, osteology Összefoglalás: A varjúfélék a legnagyobb termetű, Európában is elterjedt énekesmadarak. A kontinens madárfau- nájában a neogén elejétől, a miocénból ismertek, és jelenleg 11 fajjal vannak képviselve. Termetük következtében gyakrabban előfordulnak a fosszilis anyagban, mint a többi énekesmadár típus, és ennek következtében nagyobb mértékben is tanulmányozták őket. Jelen tanulmányban bemutatjuk az ismert európai evolúciójukat és fosszilis taxonjaikat, és foglalkozunk a recens fajok csonttanával is. Kulcsszavak: Corvidae, neogén, negyedidőszak, Európa, Kárpát-medence, csonttan Department of Paleontology, Eötvös Loránd University, 1117 Budapest, Pázmány Péter sétány 1/c, Hungary, e-mail: [email protected] Introduction About half of the current avian species – if not more – consists of songbirds, which are dis- tributed all around the world apart from Antarctica with a large number of specimens. -

Thailand Custom Tour 29 January -13 February, 2017
Tropical Birding Trip Report THAILAND JANUARY-FEBRUARY, 2017 Thailand custom tour 29 January -13 February, 2017 TOUR LEADER: Charley Hesse Report by Charley Hesse. Photos by Charley Hesse & Laurie Ross. All photos were taken on this tour When it comes to vacation destinations, Thailand has it all: great lodgings, delicious food, scenery, good roads, safety, value for money and friendly people. In addition to both its quantity & quality of birds, it is also one of the most rapidly evolving destinations for bird photography. There are of course perennial favourite locations that always produce quality birds, but year on year, Thailand comes up with more and more fantastic sites for bird photography. On this custom tour, we followed the tried and tested set departure itinerary and found an impressive 420 species of birds and 16 species of mammals. Some of the highlights included: Spoon-billed Sandpiper and Nordmann’s Greenshank around Pak Thale; Wreathed Hornbill, Long-tailed & Banded Broadbills inside Kaeng Krachan National Park; Rosy, Daurian & Spot-winged Starlings at a roost site just outside; Kalij Pheasant, Scaly-breasted & Bar-backed Partridges at a private photography blind nearby; Siamese Fireback and Great Hornbill plus Asian Elephant & Malayan Porcupine at Khao Yai National Park; countless water birds at Bueng Boraphet; a myriad of montane birds at Doi Inthanon; Giant Nuthatch at Doi Chiang Dao; Scarlet-faced Liocichla at Doi Ang Khang; Hume’s Pheasant & Spot-breasted Parrotbill at Doi Lang; Yellow-breasted Buntings at Baan Thaton; and Baikal Bush-Warbler & Ferruginous Duck at Chiang Saen. It was a truly unforgettable trip. www.tropicalbirding.com +1-409-515-9110 [email protected] Tropical Birding Trip Report THAILAND JANUARY-FEBRUARY, 2017 29th January – Bangkok to Laem Pak Bia After a morning arrival in Bangkok, we left the sprawling metropolis on the overhead highways, and soon had our first birding stop at the Khok Kham area of Samut Sakhon, the neighbouring city to Bangkok. -
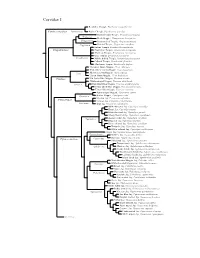
Corvidae Species Tree
Corvidae I Red-billed Chough, Pyrrhocorax pyrrhocorax Pyrrhocoracinae =Pyrrhocorax Alpine Chough, Pyrrhocorax graculus Ratchet-tailed Treepie, Temnurus temnurus Temnurus Black Magpie, Platysmurus leucopterus Platysmurus Racket-tailed Treepie, Crypsirina temia Crypsirina Hooded Treepie, Crypsirina cucullata Rufous Treepie, Dendrocitta vagabunda Crypsirininae ?Sumatran Treepie, Dendrocitta occipitalis ?Bornean Treepie, Dendrocitta cinerascens Gray Treepie, Dendrocitta formosae Dendrocitta ?White-bellied Treepie, Dendrocitta leucogastra Collared Treepie, Dendrocitta frontalis ?Andaman Treepie, Dendrocitta bayleii ?Common Green-Magpie, Cissa chinensis ?Indochinese Green-Magpie, Cissa hypoleuca Cissa ?Bornean Green-Magpie, Cissa jefferyi ?Javan Green-Magpie, Cissa thalassina Cissinae ?Sri Lanka Blue-Magpie, Urocissa ornata ?White-winged Magpie, Urocissa whiteheadi Urocissa Red-billed Blue-Magpie, Urocissa erythroryncha Yellow-billed Blue-Magpie, Urocissa flavirostris Taiwan Blue-Magpie, Urocissa caerulea Azure-winged Magpie, Cyanopica cyanus Cyanopica Iberian Magpie, Cyanopica cooki Siberian Jay, Perisoreus infaustus Perisoreinae Sichuan Jay, Perisoreus internigrans Perisoreus Gray Jay, Perisoreus canadensis White-throated Jay, Cyanolyca mirabilis Dwarf Jay, Cyanolyca nanus Black-throated Jay, Cyanolyca pumilo Silvery-throated Jay, Cyanolyca argentigula Cyanolyca Azure-hooded Jay, Cyanolyca cucullata Beautiful Jay, Cyanolyca pulchra Black-collared Jay, Cyanolyca armillata Turquoise Jay, Cyanolyca turcosa White-collared Jay, Cyanolyca viridicyanus -

Cyanocitta Cristata) in Canada, with Comments on the Genus
Proc. Helminthol. Soc. Wash. 53(2), 1986, pp. 270-276 Pseudaprocta samueli sp. n. (Nematoda: Aproctoidea) from the Blue Jay (Cyanocitta cristata) in Canada, with Comments on the Genus CHERYL M. BARTLETT' AND ROY C. ANDERSON Department of Zoology, College of Biological Science, University of Guelph, Guelph, Ontario NIG 2 W1, Canada ABSTRACT: Pseudaprocta samueli sp. n. is described from the thoracic air sac of 1 of 20 blue jays (Cyanocitta cristata (L.)) collected near Guelph, Ontario, Canada and this apparently represents the first report in the literature of Pseudaprocta in the New World. Specimens of Pseudaprocta, unidentifiable to species, from a blue jay in Alabama, U.S.A., had, however, been deposited in the United States National Museum in 1954. Pseudaprocta samueli belongs to that group of species within the genus that have short spicules (<500 jim) and 10-11 pairs of caudal papillae. It is distinguished by the irregular spacing of the cephalic spines and the presence of inflated cuticle in the caudal region of the female. An annotated list of and key to species in Pseudaprocta are given. The identities of many of the species reported in Eurasia require clarification. Aproctoids are oviparous nematode parasites Specimens of Pseudaprocta in the WHO Collabo- of the air sacs and orbital sinuses of birds. Their rating Centre for Filarioidea Collection at the Com- relationship to other superfamilies in the order monwealth Institute of Parasitology (CIP) in St. Al- bans, England, and in the Helminth Collection of the Spirurida is not well understood although an or- United States National Museum (USNM) in Beltsville, igin from Seuratoidea (Ascaridida) has been sug- Maryland, U.S.A., which were borrowed and examined gested (Chabaud, 1974; Bain and Mawson, 1981). -

SE China and Tibet (Qinghai) Custom Tour: 31 May – 16 June 2013
SE China and Tibet (Qinghai) Custom Tour: 31 May – 16 June 2013 Hard to think of a better reason to visit SE China than the immaculate cream-and-golden polka- dot spotted Cabot’s Tragopan, a gorgeous serious non-disappointment of a bird. www.tropicalbirding.com The Bar-headed Goose is a spectacular waterfowl that epitomizes the Tibetan plateau. It migrates at up to 27,000 ft over the giant Asian mountains to winter on the plains of the Indian sub-continent. Tour Leader: Keith Barnes All photos taken on this tour Introduction: SE and Central China are spectacular. Both visually stunning and spiritually rich, and it is home to many scarce, seldom-seen and spectacular looking birds. With our new base in Taiwan, little custom tour junkets like this one to some of the more seldom reached and remote parts of this vast land are becoming more popular, and this trip was planned with the following main objectives in mind: (1) see the monotypic family Pink-tailed Bunting, (2) enjoy the riches of SE China in mid-summer and see as many of the endemics of that region including its slew of incredible pheasants and the summering specialties. We achieved both of these aims, including incredible views of all the endemic phasianidae that we attempted, and we also enjoyed the stunning scenery and culture that is on offer in Qinghai’s Tibet. Other major highlights on the Tibetan plateau included stellar views of breeding Pink-tailed Bunting (of the monotypic Chinese Tibetan-endemic family Urocynchramidae), great looks at Przevalski’s and Daurian Partridges, good views of the scarce Ala Shan Redstart, breeding Black-necked Crane, and a slew of wonderful waterbirds including many great looks at the iconic Bar-headed Goose and a hoarde of www.tropicalbirding.com snowfinches. -

Zootaxa, the Type Specimens of Corvidae (Aves) In
Zootaxa 2149: 1–49 (2009) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2009 · Magnolia Press ISSN 1175-5334 (online edition) The type specimens of Corvidae (Aves) in the Museum für Naturkunde at the Humboldt-University of Berlin, with the description of a new subspecies of Dendrocitta vagabunda FRANK D. STEINHEIMER Research Associate, Science Department, Museum für Naturkunde - Leibniz Institute for Research on Evolution and Biodiversity at the Humboldt University Berlin, Invalidenstrasse 43, D-10115 Berlin, Germany & ‘Naturkundliches Universitätsmuseum’, Martin- Luther-University Halle-Wittenberg, Friedemann-Bach-Platz 6, D-06108 Halle (Saale), Germany. E-mail: [email protected] Table of contents Abstract ............................................................................................................................................................................... 2 Zusammenfassung ............................................................................................................................................................... 2 Introduction ......................................................................................................................................................................... 3 List of type specimens ......................................................................................................................................................... 3 L. [Lanius] scapulatus M. H. C. Lichtenstein, 1823 .......................................................................................................... -

A Taxonomic Revision of the New World Jays
A TAXONOMIC REVISION OF THE NEW WORLD JAYS JOHN WILLIAM HARDY Moore Laboratory of Zoology Occidental College Los Angeles, California 90041 The generic and sub-generic classification of list of North American birds (including Mex- the New World jays employed by Blake (in ice and Central America), I feel it necessary Mayr and Greenway X%2:266-228) perpetu- to state my views formally on the taxonomy ates a system largely similar to that of the of New World jays, even though the habits of earliest authoritative works on New World many species in the assemblage are still poorly birds (e.g., Sharpe 1877). Blake does sub- known. merge Uroleuca Temminck and Xanthoura The following annotated arrangement ex- Nelson, which were still recognized by Hell- cludes’ the genera Gymnorhinus and Perisoreus, mayr (1934:29,30) but does not follow recom- which I do not consider to be a part of the mendations for mass lumping of genera pro- New World assemblage otherwise considered posed by Amadon ( 1944a). Phillips et al. here. The former contains only one species, (1964:106) have since also recommended which is not a jay at all by any standard except large scale amalgamation of genera in this that its plumage is blue in color. It is probably assemblage. Amadons’ recommendations were derived from Old World corvines such as premature; information on these jays gathered Nucifraga (Hardy 1961:113) which it re- and published during the past two decades sembles, except in color. Perisoreus consists of has negated some of his conclusions. Phillips two Asiatic species, one (infaustus) wide- et al. -

P0665-P0680.Pdf
A FEEDING ADAPTATION OF THE JAW ARTICULATION IN NEW WORLD JAYS (CORVIDAE) RICHARD L. ZUSI NationalMuseum of Natural History,Smithsonian Institution, Washington, D.C. 20560 USA ABSTRACT.--Thejaw articulationof mostendemic New World jays(Corvidae) has a condyle of the quadrateand an opposingcotyla of the lower jaw not found in other birds. They also have well-developed meatic articular facets of the quadrate and cranium. The tip of the rhamphothecaof the lower mandible is chisel shaped.These and other featuresconstitute a functionalunit, the buttresscomplex, that bracesthe partiallyopened lower jaw and enhances its use as a chisel. The buttresscomplex stabilizes the lower jaw by anchoringthe jaw on the quadrateand reducing torque on the quadrateduring pounding. A hypothesisof pounding with the lower mandible was confirmed by field observationsof Aphelocomacoerulescens coerulescens,which stabsacorns with the lower mandible and then tears off the shell using both mandibles.This may be an unusually effective method of peeling acorns,and it differs from the techniquesused by Garrulusand Pica.The origin of the complexmay not be associated with acorn eating. A slight modification of the jaw articulation in Cyanolycaprobably rep- resentsthe evolutionary precursorof the buttresscomplex. The distribution of the complex in the Corvidae suggeststhat Cyanolycais the sistergroup of other endemic New World jays. Gymnorhinusis related to the New World jays, not to Nucifraga.An exampleof convergent evolution is provided by Hemignathuswilsoni (Drepanidinae). Received 15 August1986, accepted 22 April 1987. MANY members of the Corvidae pound hard found only in certain New World jays and are food items with the tip of the bill while holding clearly associatedin somespecies with a special the food against a firm substrate with one or technique for opening nuts, especiallyacorns. -

Corvus Splendens)
ISRAEL JOURNAL OF ZOOLOGY, Vol. 43, 1997, pp. 397-399 NOTE: FIRST RECORD OF GREAT SPOTTED CUCKOO (CLAMATOR GLANDARIUS) PARASITIZING INDIAN HOUSE CROW (CORVUS SPLENDENS) REUVEN Y OSEF International Birdwatching Center, P.O. Box 774, Elat 88I06, Israel The great spotted cuckoo (Clamator glandarius) is known to breed in northern and central Israel. The Indian house crow (Corvus splendens) invaded the Elat region about two decades ago and its population is growing rapidly. Here, I report the first case of a great spotted cuckoo nestling found in the nest of an Indian house crow. This is possibly the first documented parasitic interaction between these two species whose natural distribution does not overlap. The Indian house crow has colonized the Elat-Aqaba region in the past two decades and its ability to exploit human refuse has allowed its population to grow steadily. The first individual was observed in 1976 (Shirihai, 1996) and today the breeding population is estimated at approximately 50 pairs. This is an invading species that has spread from its original range in the Indian subcontinent (Cramp and Perrins, 1994), westward along the coast line of the Indian Ocean and the Saudi Arabian subcontinent, northward along the Red Sea, and southward along the east coast of Africa. In Elat, the species is considered a pest because of its habit of attacking humans that are either eating in the open, or pass in the vicinity of their nests during the breeding season. Because this is a species that has recently invaded the region, there are no data on their reproduction or ecology and predators are mostly undocumented. -

Notes on the Evolution in the Eurasian Jay, Garrulus Glandarius (L.) (With 2 Text-Figures)
Title Notes on the Evolution in the Eurasian Jay, Garrulus glandarius (L.) (With 2 Text-figures) Author(s) KURODA, Nagahisa Citation 北海道大學理學部紀要, 13(1-4), 72-77 Issue Date 1957-08 Doc URL http://hdl.handle.net/2115/27204 Type bulletin (article) File Information 13(1_4)_P72-77.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Notes on the Evolution in the Eurasian Jay, Garrulus glandarius CL.) By Nagahisa Kuroda (Yamashina Museum of Birds, Shibuya, Tokyo) (With 2 Text-figures) Taxonomic studies and reviews of the Eurasian Jay, Garrulus glandarius, have been made by various authors, and the species, comprising over 30 races, was classified by, for example, Andreas ('40) into eight groups. Here, I would treat them in five main groups, namely: glandarius-group (15 or more races): Europe east beyond the Urals, south to Morocco, Syria and S.W. Persia. Southern populations tend to be black-crowned and white cheeked. Three subgroups. brandti-gro11p (2 or 3 races): Siberia, Sakhalin, Hokkaido, Manchuria, Korea and N. China. ~1.Q~~artus-at~ic(lp.illus ~roup Qll!I!!2ldill,UB-leucotis sroup .' ',' .-glandarLus -~roup .' :-bispecularis-aroup .-t'Y.li:P-atncaptllus, etc. (Black-crowned) .#.#$'fo leucotis-8roup (Black-crowned) .-:-:·:-:....Brandtl group .• i~!!ii't:'-.Jgp'onicus aroup --G. lanceolatus • - filidthl Fig. 1. Dis'ributional map of the Eurasian Jay. Jour. Fac. Sci. Hokkaido Univ. Ser. VI, Zool. 13, 1957 (Prof. T. Uchida Jubilee Volume). 72 Evolution in the Eurasian Jay 73 japonicus-group (5 races): Honshiu and southward, Japan. bispecularis-group (7 races): China, S.E.Tibet (Ludlow, Ibis, 93: 554), Formosa, Indo China (Delacour), N.