CDC Guidelines for Lead Exposure in Pregnant and Lactating Women
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Test for the Post of Lecturer/ Assistant Professor
UNIVERSITY OF AGRICULTURE, FAISALABAD PAKISTAN Office of the Controller of Examinations Test for the post of Lecturer/ Assistant Professor All the candidates who applied against the post of Lecturer/Assistant Professor in the University of Agriculture Faisalabad Main Campus, sub Campus Burewala, DG Khan & Toba Tek Singh advertised through National Press with 30-12-2013 as last date for receipt of applications are hereby informed that the list of eligible candidates has been uploaded on website (www.uaf.edu.pk). Test for short listing the candidates will be conducted on 15.06.2014 at 10.00 AM in the University of Agriculture, Faisalabad. The test would comprise written paper of the relevant subject with 60 marks having the following three sections with weightage of 20 marks for each. Section-A MCQs Section-B Questions requiring only a few lines to answer Section-C Questions requiring detailed answer The Roll number slips to eligible candidates have been sent at their given address. If any candidates does not receive Roll number slip two days before the examinations he/she may contact the office of the Controller of Examinations during the office hours on the following telephones and E-mail. Telephones: 041-9201749, 041-92001742, 041-9200161-69/ Ext: 2302,2303 E-mails: [email protected] The candidates are required to bring original Roll No. Slips and original National Identity Card failing which they would not be allowed to enter the Examination Hall. Mobile Phones will not be allowed in the Examination Hall. Please make photocopy of the Roll No. Slip for future reference as the original slip would be collected in the Examination Hall. -

Blood Lead Test and Anticipatory Guidance
Guideline #6 BLOOD LEAD TEST AND ANTICIPATORY GUIDANCE RATIONALE No level of lead (Pb) in the body is recognized as safe. Lead toxicity is associated with impaired cognitive, motor, behavioral, and physical abilities. In 2012, the Centers for Disease Control and Prevention (CDC) recognized a risk of neurodevelopmental sequelae at blood lead levels (BLLs) below 5 micrograms of lead per deciliter of blood (mcg/dL) and replaced the former blood lead “level of concern” (10 mcg/dL) with a “reference level” of 5 mcg/dL.1 The Childhood Lead Poisoning Prevention Branch (CLPPB) of the California Department of Public Health (CDPH) is more protective in defining increased lead exposure. It interprets the reference level to include all BLLs equal to or greater than 4.5 mcg/dL.* Lead is a common environmental contaminant present in all areas of the United States, and all children are at risk for lead’s toxic effects. Within the United States approximately half a million children ages 1-5 years have blood lead levels greater than 5 mcg/dL.2 Lead exposure is one of the most common and preventable environmental diseases among California children. Many common items are associated with lead poisoning, either as the primary source or as part of the child’s cumulative exposure. Some of these have received a good deal of public attention. However, exposure to deteriorated lead-based paint and lead- contaminated dust and soil remain the major causes of childhood lead poisoning in California. Drinking water can contain lead, although it has not been found to be a major source of lead exposure in our state. -

Environmental Policy As Social Policy? the Impact of Childhood Lead Exposure on Crime
NBER WORKING PAPER SERIES ENVIRONMENTAL POLICY AS SOCIAL POLICY? THE IMPACT OF CHILDHOOD LEAD EXPOSURE ON CRIME Jessica Wolpaw Reyes Working Paper 13097 http://www.nber.org/papers/w13097 NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 May 2007 I would especially like to thank Lawrence Katz for valuable advice, as well as David Cutler, Leemore Dafny, Amy Finkelstein, Claudia Goldin, Nora Gordon, Christopher Jencks, Walter Nicholson, Linda Reyes, René Reyes, Steven Rivkin, Geoffrey Woglom, Justin Wolfers, two anonymous referees, and many seminar participants. Numerous individuals at government agencies and petroleum industry companies generously provided data on lead in gasoline. Brent Mast, Bomy Hong, and John Indellicate II provided excellent research assistance. Any remaining errors are my own. This research was supported by grants from the National Science Foundation, the National Bureau of Economic Research, the National Institute on Aging, and the Harvard Multidisciplinary Program in Inequality and Social Policy. The views expressed herein are those of the author(s) and do not necessarily reflect the views of the National Bureau of Economic Research. © 2007 by Jessica Wolpaw Reyes. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Environmental Policy as Social Policy? The Impact of Childhood Lead Exposure on Crime Jessica Wolpaw Reyes NBER Working Paper No. 13097 May 2007 JEL No. I18,K49,Q53,Q58 ABSTRACT Childhood lead exposure can lead to psychological deficits that are strongly associated with aggressive and criminal behavior. In the late 1970s in the United States, lead was removed from gasoline under the Clean Air Act. -

Childhood Lead Exposures: Medical Management Recommendations For
Childhood Lead Exposure and Poisoning Medical Management Recommendations . There is no safe level of lead in the blood. The CDC reference for an elevated blood lead level (BLL) is 5 µg/dL. Exposure to lead can have a wide range of effects on a child’s development and behavior. Any BLL of 5 µg/dL or higher requires intervention to prevent further lead exposure and increase in blood lead levels. Any capillary blood lead level ≥5 µg/dL should be confirmed with a venous blood lead test. Recommended Schedule for Obtaining Confirmatory Venous Test After Capillary Test Capillary BLL Confirm Capillary Test with Venous Blood Test: 0 – <5 µg/dL No confirmation needed. Repeat test according to DHHS Blood Lead Screening Plan. 5 – 9 µg/dL Within 3 months* 10 – 44 µg/dL Within 1 month* 45 – 69 µg/dL Within 24 - 48 hours* ≥ 70 µg/dL Immediately as an emergency test* *The higher the BLL on a screening test, the more urgent the need for confirmatory testing. Medical Management Recommendations for Confirmed Blood Lead Levels Confirmed Follow-up Venous Recommended Actions Based on Confirmed Venous BLL BLL Test Schedule Review lab results with family. For reference, the geometric mean blood lead level for No follow-up needed. children 1-5 years old is less than 2 µg/dL. Continue to test Repeat blood lead level in 6-12 months if the child is at high risk or risk changes during < 5 µg/dL according to DHHS the timeframe. Blood Lead Screening Plan Provide anticipatory guidance and discuss common lead exposure sources. -

Lead in Drinking Water and Human Blood Lead Levels In the United States
Morbidity and Mortality Weekly Report Supplement / Vol. 61 August 10, 2012 Lead in Drinking Water and Human Blood Lead Levels in the United States U.S. Department of Health and Human Services Centers for Disease Control and Prevention Supplement CONTENTS Introduction ...........................................................................................................1 Background ............................................................................................................1 Historical Trends in Blood Lead Levels ..........................................................3 Lead in the Environments of Children ..........................................................4 Lead in Drinking Water .......................................................................................4 Conclusion ..............................................................................................................7 References ...............................................................................................................7 The MMWR series of publications is published by the Office of Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services, Atlanta, GA 30333. Suggested Citation: Centers for Disease Control and Prevention. [Title]. MMWR 2012;61(Suppl; August 10, 2012):[inclusive page numbers]. Centers for Disease Control and Prevention Thomas R. Frieden, MD, MPH, Director Harold W. Jaffe, MD, MA, Associate Director for Science James -
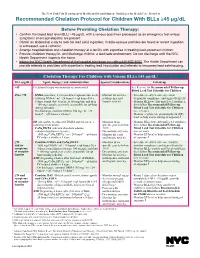
Recommended Chelation Protocol for Children with Blls ≥45 Μg/Dl
The New York City Department of Health and Mental Hygiene Guidelines for Health Care Providers Recommended Chelation Protocol for Children With BLLs ≥45 μg/dL Before Providing Chelation Therapy: • Confirm the blood lead level (BLL) ≥45 μg/dL with a venous specimen processed as an emergency test unless symptoms of encephalopathy are present. • Obtain an abdominal x-ray to look for lead solid ingestion; if radio-opaque particles are found or recent ingestion is witnessed, use a cathartic. • Arrange hospitalization and chelation therapy at a facility with expertise in treating lead-poisoned children. • Provide chelation therapy in, and discharge child to, a lead-safe environment. Do not discharge until the NYC Health Department inspects the home. • Inform the NYC Health Department of the hospital admission by calling 646-632-6002. The Health Department can provide referrals to providers with expertise in treating lead intoxication and referrals to temporary lead-safe housing. Chelation Therapy For Children with Venous BLLs ≥45 μg/dL1 BLL (μg/dL) Agent, Dosage,* and Administration Special Considerations Follow-up <45 Chelation therapy not routinely recommended See Reverse for Recommended Follow-up Blood Lead Test Schedule for Children 45 to <70 • DMSA (succimer, 2,3-meso-dimercaptosuccinic acid) • Monitor for anemia, • Schedule weekly health care visits • 1050 mg DMSA / m2 / 24 hours* ÷ q8 hours PO x neutropenia, and to monitor compliance and signs of toxicity. 5 days; round dose to nearest 100 mg/day, and then hepatic toxicity. • Monitor BLLs weekly until level stabilizes, ÷ 100-mg capsules as evenly as possible for q8-hour then follow Recommended Follow-up dosing schedule. -

Environmental Health Perspectives
ENVIRONMENTAL HEALTH ehp PERSPECTIVES ehponline.org A Geospatial Analysis of the Effects of Aviation Gasoline on Childhood Blood Lead Levels Marie Lynn Miranda, Rebecca Anthopolos, Douglas Hastings http://dx.doi.org/10.1289/ehp.1003231 Online 13 July 2011 National Institutes of Health U.S. Department of Health and Human Services Page 1 of 21 1 A Geospatial Analysis of the Effects of Aviation Gasoline on Childhood Blood Lead Levels Marie Lynn Miranda *, Rebecca Anthopolos, and Douglas Hastings Children’s Environmental Health Initiative, Nicholas School of the Environment, Duke University, Durham, NC 27708 *Corresponding author: Marie Lynn Miranda, Ph.D. Nicholas School of the Environment Duke University Box 90328 Levine Science Research Center Room A134 Durham, NC 27708-0328 919-613-8023 (o) 919-684-3227 (fax) [email protected] Page 2 of 21 2 Running Title: Aviation Gasoline and Childhood Blood Lead Levels Keywords: Aviation gasoline; Avgas; Blood lead; Childhood; Geospatial; Lead poisoning Acknowledgments: The authors gratefully acknowledge aviation gas data provided by Marion Hoyer from the EPA’s Office of Transportation and Air Quality and childhood lead exposure data provided by Ed Norman and Tena Ward from the NC Department of Environment and Natural Resources. We also acknowledge the geocoding work undertaken by the GIS staff of the Children’s Environmental Health Initiative. This work was funded in part by a grant from the USEPA (RD- 83329301) and the North Carolina Department of Environment and Natural Resources (1 H64 EH000151). Competing Financial Interest Declaration: The authors have no competing financial interests with regard to this manuscript. Abbreviations (as defined in the text): Avgas Aviation gasoline CDC Centers for Disease Control and Prevention CI Confidence interval EPA Environmental Protection Agency GIS Geographic Information Systems LL Low-lead NHANES National Health and Nutrition Examination Survey Page 3 of 21 3 Abstract Background: Aviation gasoline, commonly referred to as avgas, is a leaded fuel used in small aircraft. -

Medical and Nursing Specialists, Physicians, and Physician Assistants Handbook
MEDICAL AND NURSING SPECIALISTS, PHYSICIANS, AND PHYSICIAN ASSISTANTS HANDBOOK TEXAS MEDICAID PROVIDER PROCEDURES MANUAL: VOL. 2 JUNE 2021 TEXAS MEDICAID PROVIDER PROCEDURES MANUAL: VOL. 2 JUNE 2021 MEDICAL AND NURSING SPECIALISTS, PHYSICIANS, AND PHYSICIAN ASSISTANTS Table of Contents 1 General Information . 12 1.1 Payment Window Reimbursement Guidelines for Services Preceding an Inpatient Admission. .12 2 Chiropractic Manipulative Treatment (CMT) . .13 2.1 Enrollment . .13 2.2 Services, Benefits, Limitations, and Prior Authorization. .13 2.2.1 Prior Authorization . 14 2.3 Documentation Requirements . .14 2.4 Claims Filing and Reimbursement . .14 2.4.1 Claims Information . 14 2.4.2 Reimbursement . 14 3 Certified Nurse Midwife (CNM) . .15 3.1 Provider Enrollment. .15 3.1.1 Enrollment in Texas Health Steps (THSteps) . 16 3.2 Services, Benefits, Limitations, and Prior Authorization. .16 3.2.1 Deliveries . 16 3.2.2 Newborn Services . 16 3.2.3 Prenatal and Postpartum Services. 16 3.2.4 Laboratory and Radiology Services . 16 3.2.5 Prior Authorization . 16 3.2.6 Documentation Requirements . 17 3.2.7 Claims Filing and Reimbursement. 17 4 Certified Registered Nurse Anesthetist (CRNA) . .18 4.1 Enrollment . .18 4.2 Services, Benefits, Limitations, and Prior Authorization. .18 4.2.1 Prior Authorization . 19 4.3 Documentation Requirements . .19 4.4 Claims Filing and Reimbursement . .19 4.4.1 Claims Information . 19 4.4.1.1 Interpreting the R&S Report. 19 4.4.2 Reimbursement . 19 5 Geneticists . .20 5.1 Enrollment . .20 5.1.1 Geneticists . 20 5.2 Services, Benefits, Limitations, and Prior Authorization. .21 5.2.1 Family History . -

Ending Child Lead Exposure in Kent County
Kent County Land Bank Authority Review Subcommittee ENDING CHILD LEAD EXPOSURE IN KENT COUNTY Kent County Lead Task Force January 2018 May 11, 2017 The mission of Kent County government is to be an effective and efficient steward in delivering quality services for our diverse community. Our priority is to provide mandated services, which may be enhanced and supplemented by additional services to improve the quality of life for all our citizens within the constraints of sound fiscal policy. TABLE OF CONTENTS EXECUTIVE SUMMARY ..............................................................................3 KENT COUNTY LEAD TASK FORCE: PURPOSE AND PEOPLE .............7 GLOSSARY..................................................................................................9 Chapter 1: LEAD: SO USEFUL, SO DANGEROUS .................................10 Why people use lead and how lead can harm them More Kent County children are being poisoned by lead Chapter 2: SOURCES OF LEAD IN KENT COUNTY ...............................18 It’s not our municipal water system Lead dust in homes and soils Other lead contamination sources Young children are most at risk Chapter 3: CURRENT MODEL: TEST ONLY SOME CHILDREN ............27 Who gets tested and how Treatment and follow up for lead-poisoned children Secondary treatment vs. primary prevention Chapter 4: CURRENT MODEL: GET LEAD OUT OF POISONED CHILDREN’S HOMES ...............................................................................34 Levels of lead hazard control Who finances lead hazard control -

Care of Infants and Children
AAFP Reprint No. 260 Recommended Curriculum Guidelines for Family Medicine Residents Care of Infants and Children This document was endorsed by the American Academy of Family Physicians (AAFP). Introduction This AAFP Curriculum Guideline defines a recommended training strategy for family medicine residents. Attitudes, behaviors, knowledge, and skills that are critical to family medicine should be attained through longitudinal experience that promotes educational competencies defined by the Accreditation Council for Graduate Medical Education (ACGME), www.acgme.org. The family medicine curriculum must include structured experience in several specified areas. Much of the resident’s knowledge will be gained by caring for ambulatory patients who visit the family medicine center, although additional experience gained in various other settings (e.g., an inpatient setting, a patient’s home, a long-term care facility, the emergency department, the community) is critical for well-rounded residency training. The residents should be able to develop a skillset and apply their skills appropriately to all patient care settings. Structured didactic lectures, conferences, journal clubs, and workshops must be included in the curriculum to supplement experiential learning, with an emphasis on outcomes-oriented, evidence-based studies that delineate common diseases affecting patients of all ages. Patient-centered care, and targeted techniques of health promotion and disease prevention are hallmarks of family medicine and should be integrated in all settings. Appropriate referral patterns, transitions of care, and the provision of cost- effective care should also be part of the curriculum. Program requirements specific to family medicine residencies may be found on the ACGME website. Current AAFP Curriculum Guidelines may be found online at 1 www.aafp.org/cg. -

Puberty in Crisis
|Puberty in Crisis Puberty has long been recognised as a difficult and upsetting process for individuals and families, but it is now also being widely described as in crisis. Reportedly occurring earlier and earlier as each decade of the twenty-first century passes, sexual development now heralds new forms of temporal trouble in which sexuality, sex/gender and reproduction are all at stake. Many believe that children are growing up too fast and becoming sexual too early. Clinicians, parents and teachers all demand something must be done. Does this out-of-time development indicate that children’s futures are at risk or that we are entering a new era of environmental and social perturbation? Engaging with a diverse range of contemporary femi- nist and social theories on the body, biology and sex, Celia Roberts urges us to refuse a discourse of crisis and to rethink puberty as a combination of biological, psychological and social forces. CELIA ROBERTS is Senior Lecturer and Co-director of the Centre for Gender and Women’s Studies in the Department of Sociology at Lancaster University. She is a long-standing editor of the journal Feminist Theory and author of Messengers of Sex: Hormones, Biomedicine and Feminism (Cambridge, 2007). Puberty in Crisis: The Sociology of Early |Sexual Development celia roberts University Printing House, Cambridge CB2 8BS, United Kingdom Cambridge University Press is part of the University of Cambridge. It furthers the University’s mission by disseminating knowledge in the pursuit of education, learning and research at the highest international levels of excellence. www.cambridge.org Information on this title: www.cambridge.org/9781107104723 © Celia Roberts 2015 This publication is in copyright. -

NSEP 2014 Annual Report
NATIONAL SECURITY EDUCATION PROGRAM 2014 ANNUAL REPORT BOREN SCHOLARSHIPS AND FELLOWSHIPS ENGLISH FOR HERITAGE LANGUAGE SPEAKERS LANGUAGE TRAINING CENTERS NATIONAL LANGUAGE SERVICE CORPS ADVANCED FLAGSHIP LANGUAGE INITIATIVE PROJECT GLOBAL OFFICER THE LANGUAGE FLAG SHIP The estimated cost of this report or study for the Department of Defense is approximately $16,000 in Fiscal Years 2014 - 2015. This includes $7,000 in expenses and $9,230 in DoD labor. Generated on 2015Jan15 RefID: C-E90A501 BOREN ALUMNI QUOTES The National Security Education Program (NSEP) “The Boren Fellowship gave me insight into partnered with the Center for Naval Analyses federal opportunities that I had not previously (CNA) in 2014 to examine the Boren Scholarships considered.” and Fellowships program: identifying where alumni currently work, the influence of the Boren “The Boren Fellowship was extremely influential in program on their career paths, and how their my life. I would have been unable to spend an careers have developed since completing their entire year abroad in an immersion program.” service requirements. To gather input from Boren alumni, CNA developed and fielded a survey to “I became just the sort of international security Boren Scholars and Fellows who have completed professional that the Boren seeks to cultivate. I their service requirement. They subsequently am immensely grateful for the opportunity.” analyzed the data collected and produced a final report. The following anonymous comments “The collaborations I established as a Boren were left by alumni at the conclusion of their Fellow during grad school have lasted for 15 survey responses, and are also published in CNA’s years and are continuing.” report.1 “The fellowship I received was truly an amazing “My Boren Scholarship experience was formative.