Serum 1,5-Anhydroglucitol When Used with Fasting Plasma Glucose
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
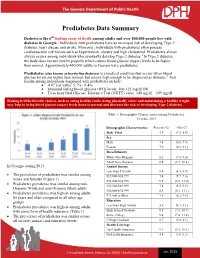
2015 Prediabetes Data Summary
The Georgia Department of Public Health Prediabetes Data Summary Diabetes is the 6th leading cause of death among adults and over 800,000 people live with diabetes in Georgia.1 Individuals with prediabetes have an increased risk of developing Type 2 diabetes, heart disease and stroke. Moreover, individuals with prediabetes often possess cardiovascular risk factors such as hypertension, obesity and high cholesterol. Prediabetes almost always occurs among individuals who eventually develop Type 2 diabetes.2 In Type 2 diabetes, the body does not use insulin properly which causes blood glucose (sugar) levels to be higher than normal. Approximately 450,000 adults in Georgia have prediabetes. Prediabetes (also known as borderline diabetes) is a medical condition that occurs when blood glucose levels are higher than normal, but are not high enough to be diagnosed as diabetes.3 Test results among individuals diagnosed with prediabetes include: A1C test value: 5.7% - 6.4% Impaired fasting blood glucose (IFG) levels: 100-125 mg/dl OR Two- hour Oral Glucose Tolerance Test (OGTT) value: 140 mg/dl – 199 mg/dl Making healt hy lifestyle choices, such as eating healthy foods, being physically active and maintaining a healthy weight, may help to bring blood glucose (sugar) levels down to normal and decrease the risk of developing Type 2 diabetes Table 1. Demographic Characteristics among Prediabetics, Georgia, 2013 Demographic Characteristics Percent (%) 95% CI State Total 7.9 (7.2, 8.9) Sex Male 7.8 (6.8, 9.5) Female 7.9 (6.9, 9.1) Race/Ethnicity -

A History of Diabetes NYSNA Continuing Education the New
A History of Diabetes NYSNA Continuing Education The New York State Nurses Association is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation. This course has been awarded 1.2 contact hours. All American Nurses Credentialing Center (ANCC) accredited organizations' contact hours are recognized by all other ANCC accredited organizations. Most states with mandatory continuing education requirements recognize the ANCC accreditation/approval system. Questions about the acceptance of ANCC contact hours to meet mandatory regulations should be directed to the Professional licensing board within that state. NYSNA has been granted provider status by the Florida State Board of Nursing as a provider of continuing education in nursing (Provider number 50-1437). A History of Diabetes 1 © 2004 NYSNA, all rights reserved. Material may not be reproduced without written permission. How to Take This Course Please take a look at the steps below; these will help you to progress through the course material, complete the course examination and receive your certificate of completion. 1. REVIEW THE OBJECTIVES The objectives provide an overview of the entire course and identify what information will be focused on. Objectives are stated in terms of what you, the learner, will know or be able to do upon successful completion of the course. They let you know what you should expect to learn by taking a particular course and can help focus your study. 2. STUDY EACH SECTION IN ORDER Keep your learning "programmed" by reviewing the materials in order. This will help you understand the sections that follow. -

Comparative Evaluation of Fructosamine and Hba1c As a Marker of Glycemic Control in Type 2 Diabetes: a Hospital Based Study
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Comparative Evaluation of Fructosamine and HbA1c as a Marker of Glycemic Control in Type 2 Diabetes: A Hospital Based Study Dr. Jyoti Goyal1, Dr. Nibhriti Das2, Dr. Navin Kumar3, Ms. Seema Raghav4, Dr. Paramjeet Singh Bhatia5, Dr. Karunesh Prasad Singh6, Dr. Sabari Das7 1DNB, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, 2Ex-Director of Laboratory services and Additional Dean Research and Academics, Nayati Healthcare and Research Centre, Mathura, India- 281003, 3Ph.D, Biostatistitian, Department of Biostatistics, Nayati Healthcare and Research Centre, Mathura, India-281003. 4M.Sc., Certified Diabetes Educator, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 5MD, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 6MD Physician, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 7Department of Laboratory Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, Corresponding Author: Dr. Jyoti Goyal ABSTRACT Introduction: Management of type 2 diabetes revolves around achievement of target glycemic control with the help of antidiabetic drugs or insulin. There are various markers for measurement of glyceamic control like HbA1c, Mean Blood Glucose and fructosamine levels. Though HbA1c is a well validated standard method for assessment of glycemic control but it has also got certain limitations. Fructosamine, a less explored method may be used as an alternative marker for an assessment of glycemic control in cases where HbA1c is unreliable or unavailable. The objective of this study is to compare the fructosamine levels with HbA1c in assessment of glycemic control in type 2 diabetics so as to assess the utility of fructosamine as an alternative marker for evaluation of glucose control. -

Acute Renal Failure in Patients with Type 1 Diabetes Mellitus G
Postgrad Med J: first published as 10.1136/pgmj.70.821.192 on 1 March 1994. Downloaded from Postgrad Med J (1994) 70, 192- 194 C) The Fellowship of Postgraduate Medicine, 1994 Acute renal failure in patients with type 1 diabetes mellitus G. Woodrow, A.M. Brownjohn and J.H. Turney Renal Unit, Leeds General Infirmary, Great George Street, Leeds LSJ 3EX, UK Summary: Acute renal failure (ARF) is a serious condition which still carries a mortality of around 50%. People with diabetes may be at increased risk of developing ARF, either as a complication of diabetic ketoacidosis or hyperosmolar coma, increased incidence of cardiovascular disease, or due to increased susceptibility ofthe kidney to adverse effects in the presence ofunderlying diabetic renal disease. During the period 1956-1992, 1,661 cases of ARF have been treated at Leeds General Infirmary. Of these, we have identified 26 patients also having type 1 diabetes. ARF due to diabetic ketoacidosis is surprisingly uncommon (14 cases out of 23 patients whose notes were reviewed). All cases of ARF complicating ketoacidosis in the last decade have been associated with particularly severe illness requiring intensive care unit support, rather than otherwise 'uncomplicated' ketoacidosis. We discuss the conditions that may result in ARF in patients with diabetes and the particular difficulties that may be encountered in management. Introduction People with diabetes may be at increased risk of Results developing acute renal failure (ARF). Acute pre- copyright. renal failure may occur as a result ofthe severe fluid Of 23 patients with type 1 diabetes complicated by depletion associated with diabetic ketoacidosis and ARF, diabetic ketoacidosis was the main underly- non-ketotic hyperosmolar coma. -

Metformin for Prediabetes
FACT SHEET FOR PATIENTS AND FAMILIES Metformin for Prediabetes What is metformin? Metformin [met-FORE-min] is a medication that is often prescribed for prediabetes in order to help prevent type 2 diabetes. It is taken by mouth (orally) as a pill. Like other prediabetes medications, it works best when you follow the rest of your treatment plan, like improving your nutrition by eating more fruits and vegetables, being physically active every day, and in most cases, losing weight. What does it do? Metformin helps lower your blood glucose (blood sugar). It does this by: Does metformin cause hypoglycemia • Decreasing the amount of glucose released by your (low blood glucose)? liver. Less glucose enters into your bloodstream. No. Metformin doesn’t cause hypoglycemia by itself. • Increasing the ability of your muscles to use glucose But combined with other medications, vigorous for energy. As more glucose is used, more glucose exercise, or too little food, it can make your blood leaves your bloodstream. glucose drop too low. Why is metformin important Since low blood glucose can be dangerous, make sure for my health? that you and your family know the symptoms. These include feeling shaky, sweaty, hungry, and irritable. Metformin can’t cure your prediabetes. But, by If you have these symptoms, take some quick-acting helping control your blood glucose, it can help sugar. Good sources are 3 or 4 glucose tablets, a half- prevent type 2 diabetes, especially among those at cup of fruit juice or regular soda, or a tablespoon of highest risk for developing type 2 diabetes. -

Prediabetes: What Is It and What Can I Do?
Prediabetes: What Is It and What Can I Do? What is prediabetes? Weight loss can delay or prevent diabetes. Prediabetes is a condition that comes before Reaching a healthy weight can help you a lot. diabetes. It means your blood glucose levels are If you’re overweight, any weight loss, even higher than normal but aren’t high enough to be 7 percent of your weight (for example, losing called diabetes. about 15 pounds if you weigh 200), may lower your risk for diabetes. There are no clear symptoms of prediabetes. You can have it and not know it. If I have prediabetes, what does it mean? It means you might get type 2 diabetes soon or down the road. You are also more likely to get heart disease or have a stroke. The good news is that you can take steps to delay or prevent type 2 diabetes. How can I delay or prevent type 2 diabetes? You may be able to delay or prevent type 2 diabetes with: Daily physical activity, such as walking. Weight loss, if needed. Losing even a few pounds will help. Medication, if your doctor prescribes it. If you have prediabetes, these steps may bring your blood glucose to a normal range. But you are still at a higher risk for type 2 diabetes. Regular physical activity can delay or prevent diabetes. Being active is one of the best ways to delay or prevent type 2 diabetes. It can also lower your weight and blood pressure, and improve cholesterol levels. Ask your health care team about activities that are safe for you. -

Effect of Biochemical Parameters Showing Atherogenicity in Type 2 Diabetic Nephropathy
International Journal of Basic and Applied Chemical Sciences ISSN: 2277-2073 (Online) An Online International Journal Available at http://www.cibtech.org/jcs.htm 2012 Vol. 2 (4) October-December pp.26-30/Raja and Shaker Research Article EFFECT OF BIOCHEMICAL PARAMETERS SHOWING ATHEROGENICITY IN TYPE 2 DIABETIC NEPHROPATHY *G. Raja and Ivvala Anand Shaker *Department of Biochemistry, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Melmaruvathur-603319, Tamil Nadu, India *Author for Correspondence ABSTRACT Diabetic nephropathy is one of the major complications of diabetes mellitus characterized by frequent microalbuminuria, elevated arterial blood pressure, a persistent decline in glomerular filtration rate and a high risk of cardiovascular morbidity and mortality. The study comprised of 30 Diabetic mellitus (DM) with microalbuminuria patients (Group 3), 30 DM without microalbuminuria patients (group 2) compared with 30 healthy controls (Group 1). Fasting glucose, post prandial glucose, lipid profile, fructosamine and microalbuminuria were investigated in all the groups. The significant increase in serum fructosamine, fasting and post prandial glucose levels along with increased microalbuminuria observed in group 3 patients compared to group 2 and group 1 patients. Hyperglycemia, increased fructosamine and increased Cholesterol, triglycerides with decreased HDL-cholesterol levels indicates the major risk of atherogenicity. Key Words: Diabetic nephropathy, Microalbuminuria, Fructosamine and Atherogenicity INTRODUCTION Diabetes mellitus (DM) is a disease in which the hallmark feature is elevated blood glucose concentrations due to loss of insulin-producing pancreatic β-cells (type 1 diabetes) or through loss of insulin responsiveness in its target tissues (type 2 diabetes). Type 1 diabetes usually begins to manifest in childhood and early adulthood, but type 2 diabetes is typically a disease for which increased age is a risk factor (Schwarz, 2009). -

Acanthocytes in the Urine Useful Tool to Differentiate Diabetic Nephropathy from Glomerulonephritis?
Pathophysiology/Complications ORIGINAL ARTICLE Acanthocytes in the Urine Useful tool to differentiate diabetic nephropathy from glomerulonephritis? GUNNAR H. HEINE, MD MATTHIAS GIRNDT, MD of patients who are most likely to have a URBAN SESTER, MD HANS K¨OHLER, MD nondiabetic glomerulopathy and to selec- tively perform renal biopsy in this sub- group of patients. Urinary erythrocyte morphology ex- amined by phase-contrast microscopy al- OBJECTIVE — The presence of hematuria has been suggested to indicate nondiabetic ne- lows to differentiation of glomerular and phropathy in diabetic patients with proteinuria. However, hematuria is frequently found in patients with biopsy-proven diabetic glomerulosclerosis without nondiabetic nephropathy. nonglomerular bleeding (10). Glomeru- Urine microscopy allows discrimination of glomerular hematuria, which is defined as acantho- lar bleeding is indicated by urinary excre- cyturia (urinary excretion of acanthocytes, which are dysmorphic erythrocytes with vesicle-like tion of acanthocytes. Acanthocytes are protrusions), from nonglomerular hematuria. We hypothesized that acanthocyturia is an un- characteristic ring-formed erythrocytes common finding in diabetic nephropathy, which suggests the presence of a nondiabetic ne- with vesicle-shaped protrusions (Fig. 1) phropathy in diabetic patients with proteinuria. (11,12) that have been described previ- ously in the peripheral blood of patients RESEARCH DESIGN AND METHODS — Urine samples of patients with the clinical diagnosis of diabetic nephropathy (n ϭ 68), of patients with biopsy-proven glomerulonephritis suffering from certain hereditary neuro- (n ϭ 43), and of age-matched healthy control subjects (n ϭ 20) were examined by phase-contrast logical disorders (abetalipoproteinemia, microscopy for the presence of hematuria (Ն8 erythrocytes/l) and acanthocyturia. Acantho- chorea-acanthocytosis, and McLeod syn- cyturia of Ն5% (5 acanthocytes among 100 excreted erythrocytes) was classified as glomerular drome) (13). -

Diabetic Nephropathy and Microalbuminuria in Pregnant Women with Type 1 and Type 2 Diabetes Prevalence, Antihypertensive Strategy, and Pregnancy Outcome
Clinical Care/Education/Nutrition/Psychosocial Research ORIGINAL ARTICLE Diabetic Nephropathy and Microalbuminuria in Pregnant Women With Type 1 and Type 2 Diabetes Prevalence, antihypertensive strategy, and pregnancy outcome 1,2 1,2 JULIE AGNER DAMM, MD LENE RINGHOLM, MD, PHD In pregnant women with type 1 di- 1,2 1,4 BJÖRG ASBJÖRNSDÓTTIR, MD BERIT WOETMANN PEDERSEN, MD 1,2 1,2,3 abetes, nephropathy is associated with NICOLINE FOGED CALLESEN ELISABETH R. MATHIESEN, MD, DMSC 1,2 poor pregnancy outcome in terms of JONATHAN M. MATHIESEN increased rates of preeclampsia and pre- term delivery (11–13). In these women, d intrauterine growth restriction (11) oc- OBJECTIVE To evaluate the prevalence of diabetic nephropathy and microalbuminuria in curs almost twice as often as in the general pregnant women with type 2 diabetes in comparison with type 1 diabetes and to describe population (13), and in the late 1990s, pregnancy outcomes in these women following the same antihypertensive protocol. preterm delivery before 34 weeks oc- RESEARCH DESIGN AND METHODSdAmong 220 women with type 2 diabetes and curred in ;30% (13). In women with 445 women with type 1 diabetes giving birth from 2007–2012, 41 women had diabetic ne- type 1 diabetes and microalbuminuria, phropathy (albumin-creatinine ratio $300 mg/g) or microalbuminuria (albumin-creatinine ratio preterm delivery and preeclampsia are 30–299 mg/g) in early pregnancy. Antihypertensive therapy was initiated if blood pressure also frequent and serious complications $ $ 135/85 mmHg or albumin-creatinine ratio 300 mg/g. (11,13,14). RESULTSdThe prevalence of diabetic nephropathy was 2.3% (5 of 220) in women with type 2 In nonpregnant subjects with diabe- diabetes and 2.5% (11 of 445) in women with type 1 diabetes (P =1.00).Thefigures for micro- tes, inhibition of the renin angiotensin albuminuria were 4.5 (10 of 220) vs. -

Early Endothelial Dysfunction Severely Impairs Skin Blood Flow
Early Endothelial Dysfunction Severely Impairs Skin Blood Flow Response to Local Pressure Application in Streptozotocin-Induced Diabetic Mice Dominique Sigaudo-Roussel, Claire Demiot, Be´renge`re Fromy, Audrey Koı¨tka, Georges Lefthe´riotis, Pierre Abraham, and Jean Louis Saumet Pressure-induced vasodilation (PIV) is a mechanism al. (2) demonstrated a major role for vasodilators such as whereby skin blood flow increases in response to pro- the calcitonin gene–related peptide and endothelial vaso- gressive locally applied pressure. Skin blood flow in dilators such as nitric oxide (NO) and prostaglandins in response to applied pressure decreased early in diabetic animal studies. In a recent study, we observed in diabetic patients as a result of vascular and/or neural impair- patients an early decrease of skin blood flow in response ment. This study was designed to determine the effect of to locally applied pressure that could be involved in the vascular changes on PIV in 1-week streptozotocin-in- development of diabetic ulceration (3). However, it was duced diabetic mice. We assessed cutaneous microvas- cular response to local increasing pressure application unclear whether skin blood flow alteration during diabetes measured by laser Doppler flowmetry (LDF) and endo- depends directly on vascular and/or neural alterations. thelium-dependent and -independent vasodilation by Diabetes through hyperglycemia is widely known to be iontophoretic delivery of acetylcholine and sodium ni- a major factor that leads to microvascular and neural troprusside and sciatic motor nerve conduction velocity complications (4). Indeed, hyperglycemia-induced end- and morphometry. In control mice, LDF increased 34% organ damage in diabetes is associated with increased flux from baseline to 0.2 kPa external pressure, showing PIV of glucose through 1) the polyol metabolic pathway response. -

Effects of Glucose on Endothelial Function in Pregnancy and the Influence of Diabetes
EFFECTS OF GLUCOSE ON ENDOTHELIAL FUNCTION IN PREGNANCY AND THE INFLUENCE OF DIABETES A thesis submitted to The University of Manchester for the degree of PhD in the Faculty of Medical and Human Sciences 2006 Haiju Henry Chirayath School of Medicine LIST OF CONTENTS ABSTRACT 17 CHAPTER 1: INTRODUCTION 26 1.1 Diabetes Mellitus 27 1.1.1 Background 27 1.1.2 Rationale of study 28 1.1.3 Definition of diabetes 28 1.1.4 Classification of diabetes 29 1.1.5 Diagnosis of diabetes 29 1.1.6 Type 1 diabetes 30 1.1.7 Type 1 diabetes in pregnancy 31 1.1.8 Type 2 diabetes 31 1.1.9 Type 2 diabetes in pregnancy 32 1.1.10 Gestational diabetes 32 1.1.10.1 Definition and diagnosis 32 1.1.10.2 Features of gestational diabetes 35 1.1.11 Other specific types of diabetes 36 1.2 Animal models of diabetes 36 1.2.1 Animal models of type 1 diabetes 37 1.2.2 Animal models of type 2 diabetes 37 1.2.3 Animal models of diabetes in pregnancy 37 1.2.4 Advantages of animal models of diabetes 38 1.2.5 Limitations of animal models of diabetes 38 1.3 Pregnancy 39 1.3.1 Carbohydrate metabolism in pregnancy 39 1.3.2 Cardiovascular changes in pregnancy 40 1.4 Arteries 41 1.4.1 Blood flow to the fetus 41 1.4.2 Resistance arteries 41 2 1.5 The Endothelium 42 1.5.1 Endothelial function 42 1.5.2 Endothelium-dependent mediators of relaxation 43 1.5.2.1 Nitric Oxide 44 1.5.2.2 Prostacyclin 45 1.5.2.3 Endothelium Derived Hyperpolarizing Factor 46 1.5.3 Endothelium-derived vasoconstrictors 47 1.5.4 Other factors affecting endothelial function 48 1.5.4.1 Age 48 1.5.4.2 Smoking 48 1.5.4.3 -

Diabetic Retinopathy in Nigerians
Brit. J. Ophthal. (I969) 53, 652 Br J Ophthalmol: first published as 10.1136/bjo.53.10.652 on 1 October 1969. Downloaded from Diabetic retinopathy in Nigerians A study of 758 patients B. 0. OSUNTOKUN Department of Medicine, University College Hospital, Ibadan, Nigeria Diabetes mellitus is a fascinating and challenging disease in African subjects. Certain aspects of the disease in Africans, especially with regard to aetiology, clinical pattern, details of management, and the incidence of some of its complications, show striking differences compared with those in Caucasian patients. Careful study of the disease may provide information not only on the aetiology of the disease, but also on its natural history and the pathogenesis ofits complications, which may be ofworld-wide importance (Tulloch, I966). Diabetic retinopathy is one such complication. In the more developed countries such as the United States of America, diabetic retino- copyright. pathy is rapidly becoming the single most important cause of blindness among the adult population. It accounted for about I8 per cent. of blindness in some states in I957/8 (Marble, I965) and the percentage ofall blindness due to diabetic retinopathy in the USA is 8-4. There is a similar incidence of diabetic retinopathy as a cause of blindness in the United Kingdom (Winter, I960). Diabetic retinopathy is not uncommon among the Bantus in South Africa (Jackson, Goldin, and Marine, I966), whereas among Nigerians it is rare (Kinnear, I963; Greenwood and Taylor, I968). This study has been carried http://bjo.bmj.com/ out to determine accurately the incidence of retinopathy in Nigerian diabetics and to delineate the various factors which may account for its rarity.