A Review on Surface-Enhanced Raman Scattering
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

DEVELOPMENT of a RAMAN SPECTROMETER to STUDY SURFACE-ENHANCED RAMAN SCATTERING by Nandita Biswas, Ridhima Chadha, Sudhir Kapoor, Sisir K
BARC/2011/E/003 BARC/2011/E/003 DEVELOPMENT OF A RAMAN SPECTROMETER TO STUDY SURFACE-ENHANCED RAMAN SCATTERING by Nandita Biswas, Ridhima Chadha, Sudhir Kapoor, Sisir K. Sarkar and Tulsi Mukherjee Radiation & Photochemistry Division 2011 BARC/2011/E/003 GOVERNMENT OF INDIA ATOMIC ENERGY COMMISSION BARC/2011/E/003 DEVELOPMENT OF A RAMAN SPECTROMETER TO STUDY SURFACE-ENHANCED RAMAN SCATTERING by Nandita Biswas, Ridhima Chadha, Sudhir Kapoor, Sisir K. Sarkar and Tulsi Mukherjee Radiation & Photochemistry Division BHABHA ATOMIC RESEARCH CENTRE MUMBAI, INDIA 2011 BARC/2011/E/003 BIBLIOGRAPHIC DESCRIPTION SHEET FOR TECHNICAL REPORT (as per IS : 9400 - 1980) 01 Security classification : Unclassified 02 Distribution : External 03 Report status : New 04 Series : BARC External 05 Report type : Technical Report 06 Report No. : BARC/2011/E/003 07 Part No. or Volume No. : 08 Contract No. : 10 Title and subtitle : Development of a Raman spectrometer to study surface-enhanced Raman scattering 11 Collation : 31 p., 15 figs. 13 Project No. : 20 Personal author(s) : Nandita Biswas; Ridhima Chadha; Sudhir Kapoor; Sisir K. Sarkar; Tulsi Mukherjee 21 Affiliation of author(s) : Radiation and Photochemistry Division , Bhabha Atomic Research Centre, Mumbai 22 Corporate author(s) : Bhabha Atomic Research Centre, Mumbai - 400 085 23 Originating unit : Radiation and Photochemistry Division, BARC, Mumbai 24 Sponsor(s) Name : Department of Atomic Energy Type : Government Contd... BARC/2011/E/003 30 Date of submission : January 2011 31 Publication/Issue date : February 2011 40 Publisher/Distributor : Head, Scientific Information Resource Division, Bhabha Atomic Research Centre, Mumbai 42 Form of distribution : Hard copy 50 Language of text : English 51 Language of summary : English, Hindi 52 No. -
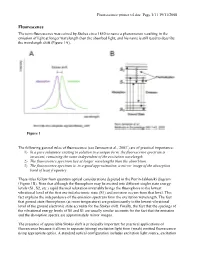
The Term Fluorescence Was Coined by Stokes Circa 1850 to Name A
Fluorescence primer v4.doc Page 1/11 19/11/2008 Fluorescence The term fluorescence was coined by Stokes circa 1850 to name a phenomenon resulting in the emission of light at longer wavelength than the absorbed light, and his name is still used to describe the wavelength shift (Figure 1A). Figure 1 The following general rules of fluorescence (see Jameson et al., 20031) are of practical importance: 1) In a pure substance existing in solution in a unique form, the fluorescence spectrum is invariant, remaining the same independent of the excitation wavelength. 2) The fluorescence spectrum lies at longer wavelengths than the absorbtion. 3) The fluorescence spectrum is, to a good approximation, a mirror image of the absorption band of least frequency These rules follow from quantum optical considerations depicted in the Perrin-Jablonski diagram (Figure 1B). Note that although the fluorophore may be excited into different singlet state energy levels (S1, S2, etc.) rapid thermal relaxation invariably brings the fluorophore to the lowest vibrational level of the first excited electronic state (S1) and emission occurs from that level. This fact explains the independence of the emission spectrum from the excitation wavelength. The fact that ground state fluorophores (at room temperature) are predominantly in the lowest vibrational level of the ground electronic state accounts for the Stokes shift. Finally, the fact that the spacings of the vibrational energy levels of S0 and S1 are usually similar accounts for the fact that the emission and the absorption spectra are approximately mirror images. The presence of appreciable Stokes shift is principally important for practical applications of fluorescence because it allows to separate (strong) excitation light from (weak) emitted fluorescence using appropriate optics. -

DXR Raman Microscope
Thermo Scientific DXR Raman Microscope Put high performance to work for you DXR Raman microscope Rethinking Raman for industry and applied research We’ve completely redefined the Raman microscope so that it lets you focus on your work rather than the tools you use to do it. Without sacrificing performance, we’ve built an instrument family around reliability, confidence, and usability. Furthermore, we’ve solved practical application problems with innovations that improve your ability to get to good information quickly. We’ve put expertise under the hood, real world problem solving into the design, and support everywhere in the world. Welcome to Thermo Scientific DXR Raman spectroscopy, Raman that works for you rather than the other way around. Packaging Forensics Fibers Gems/Geology Art Restoration Focus on your project or problem, not your instrumentation Instrumentation used in solving analytical challenges and characterizing materials in today’s fast paced, results-driven world is very different than what is required in advanced spectroscopic experiments. We designed the Thermo Scientific™ DXR™ Raman system for users who are trying to solve an engineering problem, characterize a novel material, defend a patent, or tackle a backlog of evidence. We designed an instrument that is not only powerful but considers your need for reliability, repeatability, ease of use, and assurance in results. If your analysis is a means to an end that simply requires answers quickly and confidently, the DXR microscope is without question the right Raman for your laboratory. Nanotechnology DXR Raman designed for real world problems... DXR Raman and Materials Characterization Unprecedented laser power control and patented* three path alignment assure that you are getting the best results from the right part of your material. -

Resonant Raman Spectroscopy of Nanotubes
10.1098/rsta.2004.1444 Resonant Raman spectroscopy of nanotubes By Christian Thomsen1, Stephanie Reich2 and Janina Maultzsch1 1Technische Universit¨at Berlin, Hardenbergstraße 36, 10623 Berlin, Germany 2Department of Engineering, University of Cambridge, Trumpington Street, Cambridge CB2 1PZ, UK ([email protected]) Published online 28 September 2004 Single and double resonances in Raman scattering are introduced and six criteria for the observation and identification of double resonances stated. The experimental situation in carbon nanotubes is reviewed in view of these criteria. The evidence for the D mode and the high-energy mode is found to be overwhelming for a double- resonance process to take place, whereas the nature of the radial breathing-mode Raman process remains undecided at this point. Consequences for the application of Raman scattering to the characterization of nanotubes are discussed. Keywords: carbon nanotubes; double resonance; Raman scattering; defects 1. Introduction Raman scattering in carbon nanotubes has developed into a method of choice in the investigation of their physical properties and their characterization. The study of electronic resonances in the Raman spectra, a method which has been used exten- sively, for example, in work on semiconductors (Cardona 1982), gives us a wealth of information about the electronic band structure of a material. This is also true for the Raman work on carbon nanotubes, where resonance studies have moved into the focus of research. Traditional Raman studies of carbon nanotubes focus on the radial breathing mode (RBM), a mode where all atoms vibrate in phase in the radial direction (Dresselhaus et al. 1995). Its frequency, as can easily be shown (Jishi et al. -

Simplified Near-Degenerate Four-Wave-Mixing Microscopy
molecules Article Simplified Near-Degenerate Four-Wave-Mixing Microscopy Jianjun Wang 1,† , Xi Zhang 1,†, Junbo Deng 1, Xing Hu 1, Yun Hu 1, Jiao Mao 1, Ming Ma 1, Yuhao Gao 1, Yingchun Wei 1, Fan Li 1, Zhaohua Wang 1, Xiaoli Liu 1, Jinyou Xu 2,* and Liqing Ren 1,* 1 School of Energy Engineering, Yulin University, Yulin 719000, China; [email protected] (J.W.); [email protected] (X.Z.); [email protected] (J.D.); [email protected] (X.H.); [email protected] (Y.H.); [email protected] (J.M.); [email protected] (M.M.); [email protected] (Y.G.); [email protected] (Y.W.); [email protected] (F.L.); [email protected] (Z.W.); [email protected] (X.L.) 2 South China Academy of Advanced Optoelectronics, South China Normal University, Guangzhou 510006, China * Correspondence: [email protected] (J.X.); [email protected] (L.R.) † These authors contribute equally. Abstract: Four-wave-mixing microscopy is widely researched in both biology and medicine. In this paper, we present a simplified near-degenerate four-wave-mixing microscopy (SNDFWM). An ultra-steep long-pass filter is utilized to produce an ultra-steep edge on the spectrum of a femtosecond pulse, and a super-sensitive four-wave-mixing (FWM) signal can be generated via an ultra-steep short-pass filter. Compared with the current state-of-the-art FWM microscopy, this SNDFWM microscopy has the advantages of simpler experimental apparatus, lower cost, and easier operation. We demonstrate that this SNDFWM microscopy has high sensitivity and high spatial resolution in both nanowires and biological tissues. -

Stokes Shift
Stokes shift ticular molecular structure. If a material has a direct bandgap in the range of visible light, the light shining on it is absorbed, causing electrons to become excited to a higher energy state. The electrons remain in the ex- cited state for about 10−8 seconds. This number varies over several orders of magnitude depending on the sam- ple, and is known as the fluorescence lifetime of the sam- ple. After losing a small amount of energy in some way (hence the longer wavelength), the molecule returns to the ground state and energy is emitted. 2 References Absorption and emission spectra of Rhodamine 6G with ~25 nm [1] Gispert, J.R. (2008). Coordination Chemistry. Wiley- Stokes shift VCH. p. 483. ISBN 3-527-31802-X. [2] Albani, J.R. (2004). Structure and Dynamics of Macro- Not to be confused with Stark shift. molecules: Absorption and Fluorescence Studies. Elsevier. p. 58. ISBN 0-444-51449-X. Stokes shift is the difference (in wavelength or frequency [3] Lakowicz, J.R. 1983. Principles of Fluorescence Spec- units) between positions of the band maxima of the troscopy, Plenum Press, New York. ISBN 0-387-31278- absorption and emission spectra (fluorescence and Raman 1. being two examples) of the same electronic transition.[1] [4] Guilbault, G.G. 1990. Practical Fluorescence, Second It is named after Irish physicist George G. Stokes.[2][3][4] Edition, Marcel Dekker, Inc., New York. ISBN 0-8247- When a system (be it a molecule or atom) absorbs a 8350-6. photon, it gains energy and enters an excited state. -

Fiber Amplifiers and Fiber Lasers Based on Stimulated Raman
micromachines Review Fiber Amplifiers and Fiber Lasers Based on Stimulated Raman Scattering: A Review Luigi Sirleto * and Maria Antonietta Ferrara National Research Council (CNR), Institute of Applied Sciences and Intelligent Systems, Via Pietro Castellino 111, 80131 Naples, Italy; [email protected] * Correspondence: [email protected] Received: 10 January 2020; Accepted: 24 February 2020; Published: 26 February 2020 Abstract: Nowadays, in fiber optic communications the growing demand in terms of transmission capacity has been fulfilling the entire spectral band of the erbium-doped fiber amplifiers (EDFAs). This dramatic increase in bandwidth rules out the use of EDFAs, leaving fiber Raman amplifiers (FRAs) as the key devices for future amplification requirements. On the other hand, in the field of high-power fiber lasers, a very attractive option is provided by fiber Raman lasers (FRLs), due to their high output power, high efficiency and broad gain bandwidth, covering almost the entire near-infrared region. This paper reviews the challenges, achievements and perspectives of both fiber Raman amplifier and fiber Raman laser. They are enabling technologies for implementation of high-capacity optical communication systems and for the realization of high power fiber lasers, respectively. Keywords: stimulated raman scattering; fiber optics; amplifiers; lasers; optical communication systems 1. Introduction Optical communication systems require optoelectronic devices, such as sources, detectors and so on, and utilize fiber optics to transmit the light carrying the signals impressed by modulators. Optical fibers are affected by chromatic dispersion, losses, and nonlinearity. Dispersion control is, usually, achieved via fiber geometry and material composition. Losses limit the transmission distance in modern long haul fiber-optic communication systems, so in order to boost a weak signal, optical amplifiers have been developed. -

Raman Microscope
COMPACT AND FULLY AUTOMATED RAMAN MICROSCOPE RM5 EDINBURGH INSTRUMENTS Edinburgh Instruments has been providing high performance instrumentation in the Molecular Spectroscopy market for almost 50 years. Our commitment to offering the highest quality, highest sensitivity instruments for our customers has now expanded to include the best Raman microscopes for all research and analytical requirements. As always, Edinburgh Instruments provides world-class customer support and service throughout the lifetime of our instruments. MOLECULAR SPECTROSCOPY SINCE 1971 CIRCLE Photoluminescence CIRCLE Raman CIRCLE UV-Vis CIRCLE Transient Absorption BIOSCIENCES PHARMACEUTICALS CHEMICALS POLYMERS NANO-MATERIALS RM5 RAMAN MICROSCOPE The RM5 is a compact and fully automated Raman microscope for analytical and research purposes. The truly confocal design of the RM5 is unique to the market and offers uncompromised spectral resolution, spatial resolution, and sensitivity. The RM5 builds on the expertise of robust and proven building blocks, combined with modern optical design considerations; and a focus on function, precision and speed. The result is a modern Raman microscope that stands alone in both specifications and ease of use. KEY FEATURES Truly Confocal – with variable slit and multiple position adjustable pinhole for higher image definition, better fluorescence rejection and application optimisation A MODERN RAMAN MICROSCOPE THAT Integrated Narrowband Raman Lasers – up to 3 computer- STANDS ALONE IN controlled lasers for ease of use, enhanced stability -

Tip-Enhanced Raman Spectroscopic Detection of Aptamers
Tip-enhanced Raman spectroscopic detection of aptamers Siyu He1,2, Hongyuan Li1,2, Zhe He1, and Dmitri V. Voronine1,3 1 Texas A&M University, College Station, TX 77843, USA 2 Xi’an Jiaotong University, Xi’an 710049, China 3 Baylor University, Waco, TX 76798, USA Abstract Single molecule detection, sequencing and conformational mapping of aptamers are important for improving medical and biosensing technologies and for better understanding of biological processes at the molecular level. We obtain vibrational signals of single aptamers immobilized on gold substrates using tip-enhanced Raman spectroscopy (TERS). We compare topographic and optical signals and investigate the fluctuations of the position- dependent TERS spectra. TERS mapping provides information about the chemical composition and conformation of aptamers, and paves the way to future single-molecule label-free sequencing. 1 1. Introduction Surface- and tip-enhanced Raman scattering can be used to reveal the molecular bonds as well as the functional components in biomaterials, which was applied to few and single molecule sensing1,2,3,4. Surface-enhanced Raman scattering (SERS) utilizes plasmonic resonances of metallic nanostructures to enhance Raman signals of biomolecules5. Weak Raman signals of complex biomolecules could be challenging for measurements at the single molecule level6,7. SERS can be used boost the Raman signals at the plasmonic resonance condition. Tip-enhanced Raman spectroscopy (TERS) is scanning probe microscope combined with the plasmonic enhancement of SERS and provides nanoscale label-free, direct chemical imaging method due to high lateral resolution and possibility of simultaneous imaging and sensing at the single-molecule level4,8. Recently, several DNA sensing methods have emerged in various fields of research as subjects of intense studies9,10. -

Theoretical Investigation of Stokes Shifts and Reaction Pathways
Theoretical Investigation of Stokes Shifts and Reaction Pathways by Laken M. Top - ARI IVS Associate of Arts, Science Option Yakima Valley Community College, 2007 Bachelor of Science, Chemistry and Mathematics RES University of Idaho, 2009 SUBMITTED TO THE DEPARTMENT OF CHEMISTRY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN CHEMISTRY AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY SEPTEMBER 2012 @ 2012 Massachusetts Institute of Technology. All rights reserved. 1 1-/ Signature of Author: Department of Chemistry July 12, 2012 Certified by: I Troy Van Voorhis ,1 Associate Professor of Chemistry Thesis Supervisor I / Certified by: f -y Jeffrey C. Grossman Associate Professor of Materials Science and Engineering Thesis Supervisor Accepted by: Robert W. Field Professor of Chemistry Chairman, Department Committee on Graduate Theses 1 Theoretical Investigation of Stokes Shifts and Reaction Pathways by Laken M. Top Submitted to the Department of Chemistry on July 12, 2012, in partial fulfillment of the requirements for the degree of Master of Science in Chemistry Abstract Solar thermal fuels and fluorescent solar concentrators provide two ways in which the energy from the sun can be harnessed and stored. While much progress has been made in recent years, there is still much more to learn about the way that these applications work and more efficient materials are needed to make this a feasible source of renewable energy. Theoretical chemistry is a powerful tool which can provide insight into the processes involved and the properties of materials, allowing us to predict substances that might improve the efficiency of these devices. In this work, we explore how the delta self-consistent field method performs for the calculation of Stokes shifts for conjugated dyes. -
Stokes Shift Spectroscopy Highlights Differences of Cancerous and Normal Human Tissues
3360 OPTICS LETTERS / Vol. 37, No. 16 / August 15, 2012 Stokes shift spectroscopy highlights differences of cancerous and normal human tissues Yang Pu, Wuabo Wang, Yuanlong Yang, and R. R. Alfano* Institute for Ultrafast Spectroscopy and Lasers, and Department of Physics, City College of the City University of New York, Convent Avenue at 138th Street, New York, New York 10031, USA *Corresponding author: [email protected] Received May 15, 2012; accepted June 25, 2012; posted June 28, 2012 (Doc. ID 168649); published August 6, 2012 The Stokes shift spectroscopy (S3) offers a simpler and better way to recognize spectral fingerprints of fluorophores in complex mixtures. The efficiency of S3 for cancer detection in human tissue was investigated systematically. The alterations of Stokes shift spectra (S3) between cancerous and normal tissues are due to the changes of key fluor- ophores, e.g., tryptophan and collagen, and can be highlighted using optimized wavelength shift interval. To our knowledge, this is the first time to explicitly disclose how and why S3 is superior in comparison with other conventional spectroscopic techniques. © 2012 Optical Society of America OCIS codes: 170.0170, 170.6510, 300.0300, 300.6170. Fluorescence spectroscopy has been widely investigated These differences may reflect tissue fluorophores’ for diagnosing cancer since the study by Alfano et al. in change during the evolution of cancer. In order to under- the 1980s [1]. The difference between the emission and stand which components mainly contribute to these absorption peaks is known as the Stokes shift interval, changes, S3 of the main fluorophores in breast tissue, Δλss. -

Coherent Anti-Stokes Raman Scattering with Single-Molecule Sensitivity Using a Plasmonic Fano Resonance
ARTICLE Received 13 Jan 2014 | Accepted 16 Jun 2014 | Published 14 Jul 2014 DOI: 10.1038/ncomms5424 Coherent anti-Stokes Raman scattering with single-molecule sensitivity using a plasmonic Fano resonance Yu Zhang1,2, Yu-Rong Zhen1,2, Oara Neumann2,3, Jared K. Day2,3, Peter Nordlander1,2,3 & Naomi J. Halas1,2,3 Plasmonic nanostructures are of particular interest as substrates for the spectroscopic detection and identification of individual molecules. Single-molecule sensitivity Raman detection has been achieved by combining resonant molecular excitation with large electromagnetic field enhancements experienced by a molecule associated with an inter- particle junction. Detection of molecules with extremely small Raman cross-sections (B10 À 30 cm2 sr À 1), however, has remained elusive. Here we show that coherent anti-Stokes Raman spectroscopy (CARS), a nonlinear spectroscopy of great utility and potential for molecular sensing, can be used to obtain single-molecule detection sensitivity, by exploiting the unique light harvesting properties of plasmonic Fano resonances. The CARS signal is enhanced by B11 orders of magnitude relative to spontaneous Raman scattering, enabling the detection of single molecules, which is verified using a statistically rigorous bi-analyte method. This approach combines unprecedented single-molecule spectral sensitivity with plasmonic substrates that can be fabricated using top-down lithographic strategies. 1 Department of Physics and Astronomy, Rice University, Houston, Texas 77005, USA. 2 Laboratory for Nanophotonics, Rice University, Houston, Texas 77005, USA. 3 Department of Electrical and Computer Engineering, Rice University, Houston, Texas 77005, USA. Correspondence and requests for materials should be addressed to N.J.H. (email: [email protected]).