Investigations on Quality of Railway Station Drinking Water from Parbhani to Nanded Rout P
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

District Census Handbook, Parbhani, Part II
CENSUS OF INDIA, 1951 HYDERABAD STATE District Cel1sus Elandbook PARBHANI DISTRICT PART II Issued by BUREAU OF ECONOMICS AND STATISTICS FINANCE DEPAR'TMENT GOVE.RNMENT OF HYDERABAD PRICE Rs. 4 PARBHANI DISTRICT ~ ::0 .1) ;0 -t ., -i 2 ~ 0 » » Cf) c: < );> r- oo r rn r -f C -t :.J ;;u 0 c ~ ~ ~ Ii) :0 :b » 0 0 2!: -< -I -t ~ C) :r: CI) )). : 0 ~ '"» c QJ 0 2 )). Ii) c: l> ~ 0 P ":II ~ D -< 0 : -I ~ rn -(/) ;: :0 '<: Q :u-I j tt;;! l ~ 0- ~ \ -I I , .....~ 0 o@ ,: :tI .....0 til "'"I ::0 0- -f .... "P Q 'J, -0 ".p<, (l1 -o l=o :0 o J ...., -(/) I ~ • J -I _.'\.. .. , 'I ::0 .. ......_ '\., -o ... ........,... , ....... ." ..... :» ", ." l> ::0 '"o (D .... _J -< '" ("...r' -t .~. :x: ( (J)"' ~ "'-I r ,.,'"~ "'< 2 ITt -t ~ 0 R' ..,CD ~ r .x: » l> 0 » ::0 :r ~ z > -< )). o l> r- ::0 "0 m %J 0 o l> o 0 -(/) :II ", o VI o ;2; ~ -n '""-t .... CONTENTS PAGE :MAP OJ' P AB.BHANl DISTRICT Fromispiece Preface v .Explanatory Note on Tables .. 1 List of Census Tracts-Parbhani District 5 1. GENERAL POPULATION TABLES Table A- I-Area, Houses and Population .. 6 Table A- II-Variation in Population during Fifty Years 8 'Table A- III-Towns and Villages Classified by Population 10 Table A- IV-Towns Classified by PopUlation with Variations since 1901 12 'Table A- V-Towns arranged Territorially with Population by Livelihood Classes 14 2. ECONOMIC TABLES 'Table B- I-Livelihood Classes and Sub-Classes .. 16 'Table B- II-Secondary Means of Livelihood .. 22 /' 3. -
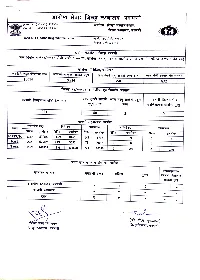
R AT: Fet 5YIT, UTYUT
R AT: fET 5YIT, UTYUT . ( R*4R) *raY4C AL fE TT, TRHt Email ID: [email protected] T.5. TB4/4ifsui/3oR? R://2o 10696 9514 350 832 221 40 2 f 87055 1189 81614 RTPCR 1337 148 4708 0 593 140 RAT 222 62759 149 56924 73 5835 Total 1559 149814 1338 138538 221 10543 593 140 R (t. Trsr) ffea, Trwft fE TA, TY¥u 7. 34T.HT J. M5.4RHUT Ro TT Tee, TTquft RO 4737.8t.TT EÍTi7 9 3 Ujt, HI DCHC 33 aATHTE TS THyE F DCHC o34tET EUITTY, TaE DCHC o Co o o o o oRHT T.HT.. R . o RoR fE 4, TYYYt fI RR/o 3/3oR 77.T R TATT TT TfAur ar.3T 3HATT 4R foEUITA, TTyut cOvID 19 POSITIVE PATIENT LIST SR NO DATE-21/03/2021 Taluka Ae Address PALAM 21 AT BHOGAON TQ.PALAM PALAM 29 AT BHOGAON TQ.PALAM PARBHANI 25 AT POST AARKHED PARBHANI 27 AT POST AARKHED GANGAKHED 5 PATHRI NARLAD GANGAKHED PURNA MASALATANDA PATHARI PURNA M DHANGAR TAKLI TQ PURNA 22 M KANTHESHWARTQ PURNA PURNA 36 DHANGAR TAKAL PURNA PURNA KAWALGAON TQ PURNA 20 PIMPRAN TQ PURNA PURNA KAWALGAONTQ PURNA PURNA KAWALGAON TQ PURNA PURNA APPIMPALA LOKHANE PURNA AP PIMPALA LOKHADE TQ.PURNA DIST.PARBHANI PURNA A/PPIMPALA LOKHADE TQ.PURNA DIST PARBHANI PURNA A/P PIMPALA LOKHADE TQ.PURNA DIST PARBHANI PURNA AP PIMPALA LOKHADE TQ.PURNA DIAT.PARBHANI PURNA KAWALGAON TQ PURNA PURNA 0 KAWALGAON TQ PURNA PARBHANI HUSEN COLLEGE PARBHANI ARBHANIT U PIMPLEGAO PARBHANI JINTUR V BHOGAON JINTUR PARBHANI 6 NEW MONDHA PARBHANI 25 PARBHANI 19 LOKMANYANAGAR PARBHANI 26 PARBHANI 29 M MOGRA NAGAR PARBHANI 27 PARBHANI PRABHAVATI NAGER PARBHANI 28 PARBHANI 21 M VIL HOSPITAL PARBHANI 29 JINTUR 2 M JINTURDIST PARBHANI 30 PARBHANI1 -

POCRA Villages Phase 2
POCRA Villages Phase 2 Sr. District Subdivision Taluka Cluster Code Census Village No. Code 1 Akola Akola Akola 501_ptr-1_03 529995 Agar 2 Akola Akola Akola 501_ptr-2_03 530009 Amanatpur 3 Akola Akola Akola 501_ptr-1_03 530004 Takoda 4 Akola Akola Akola 501_ptr-1_03 529998 Badlapur 5 Akola Akola Akola 501_ptr-2_03 529999 Bhod 6 Akola Akola Akola 501_ptr-2_03 530126 Bhaurad 7 Akola Akola Akola 501_ptk-1_01 530073 Tankhed 8 Akola Akola Akola 501_ptr-3_08 530150 Chandur 9 Akola Akola Akola 501_ptr-2_03 530125 Dabki 10 Akola Akola Akola 501_ptsb-1_03 530022 Dahihanda 11 Akola Akola Akola 501_pt-18_01 529974 Dhamana 12 Akola Akola Akola 501_ptr-4_04 529985 Dudhala 13 Akola Akola Akola 501_ptr-4_04 529984 Mandala 14 Akola Akola Akola 501_pt-18_01 529978 Gandhigram 15 Akola Akola Akola 501_pt-18_01 529977 Gopalkhed 16 Akola Akola Akola 501_ptsp-1_05 530019 Ganori 17 Akola Akola Akola 501_ptsp-1_05 530021 Hingni bk (dahihanda) 18 Akola Akola Akola 501_ptsp-1_05 530020 Khanapur 19 Akola Akola Akola 501_ptr-1_03 529996 Kanchanpur 20 Akola Akola Akola 501_pt-19_03 530025 Kapileshwar 21 Akola Akola Akola 501_pts-1_05 530014 Kati 22 Akola Akola Akola 501_pts-1_05 530015 Pati 23 Akola Akola Akola 501_pt-19_03 530023 Katyar 24 Akola Akola Akola 501_ptk-1_01 530071 Khadka 25 Akola Akola Akola 501_ptr-2_04 530003 Khadki takali 26 Akola Akola Akola 501_ptr-4_04 529983 Khambora 27 Akola Akola Akola 501_ptr-2_02 530148 Kharab kh 28 Akola Akola Akola 501_ptk-1_01 530081 Pahadpur 29 Akola Akola Akola 501_ptr-1_02 529987 Hatla 30 Akola Akola Akola 501_ptr-1_02 -

Speech of Mamata Banerjee Introducing the Railway Budget 2011-12 25Th February 2011
Speech of Mamata Banerjee introducing the Railway Budget 2011-12 25th February 2011 1. Madam Speaker, I rise to present before this august House the Revised Estimates for 2010-11 and the estimated receipts and expenditure for 2011-12. I deem it an honour to present the third Railway Budget under the kind guidance of the hon'ble Prime Minister. I profusely thank the Finance Minster for his continued support and encouragement to the railways. 2. As the hon’ble members are aware, the wheels of the railways continue to move 24 hours, all 365 days. Railway’s services are comparable to emergency services, required all the time. I am proud of the 14 lakh members of my railway family, who toil day and night with unparalleled dedication. I am also grateful to all passengers without whose cooperation and consideration, we could not have run this vast system. I have also received unstinted support from our two recognised federations and staff and officers’ associations. 3. Madam, rail transportation is vitally interlinked with the economic development of the country. With the economy slated to grow at a rate of 8-9%, it is imperative that the railways grow at an even faster pace. I see the railways as an artery of this pulsating nation. Our lines touch the lives of humble people in tiny villages, as they touch the lives of those in the bustling metropolises. 4. We are taking a two-pronged approach, scripted on the one hand, by a sustainable, efficient and rapidly growing Indian Railways, and on the other, by an acute sense of social responsibility towards the common people of this nation. -

Maharashtra State Boatd of Sec & H.Sec Education Pune
MAHARASHTRA STATE BOATD OF SEC & H.SEC EDUCATION PUNE PAGE : 1 College wise performance ofFresh Regular candidates for HSC March-2018 Exam. Candidates passed College No. Name of the collegeStream Candidates Candidates Total Pass Registerd Appeared Pass UDISE No. Distin- Grade Grade Pass Percent ction I II Grade 56.01.001 DEOGIRI COLLEGE AURANGABAD RAILWAY STATION SCIENCE 1858 1854 287 908 581 7 1783 96.17 27191109505 ROAD PA ARTS 320 320 37 86 97 11 231 72.18 COMMERCE 873 873 387 286 153 9 835 95.64 HSC.VOC 139 139 3 62 51 0 116 83.45 TOTAL 3190 3186 714 1342 882 27 2965 93.06 56.01.002 MILIND COLLEGE OF SCIENCE, NAGASENVANA, SCIENCE 504 499 5 93 292 19 409 81.96 27191101009 AURANGABAD TOTAL 504 499 5 93 292 19 409 81.96 56.01.003 DR.B.A.COLLEGE ARTS COMM NAGSENVANA ARTS 18 18 0 2 1 2 5 27.77 27191101010 COMMERCE 42 42 0 5 20 2 27 64.28 TOTAL 60 60 0 7 21 4 32 53.33 56.01.004 MILIND COLLEGE OF ARTS, NAGASENVANA, ARTS 299 299 12 59 92 7 170 56.85 27191101011 AURANGABAD TOTAL 299 299 12 59 92 7 170 56.85 56.01.005 NALANDA JR.COLL.OF ARTS ARTS 32 32 0 7 13 1 21 65.62 27191109110 &COMM,RAMANAGAR,AURANGABAD COMMERCE 22 22 0 6 12 1 19 86.36 TOTAL 54 54 0 13 25 2 40 74.07 56.01.006 SHRI.SHIVAJI JR.COLLEGE, KHOKADPURA, ARTS 20 20 2 2 11 0 15 75.00 27191105905 AURANGABAD COMMERCE 19 19 1 3 10 4 18 94.73 MAHARASHTRA STATE BOATD OF SEC & H.SEC EDUCATION PUNE PAGE : 2 College wise performance ofFresh Regular candidates for HSC March-2018 Exam. -
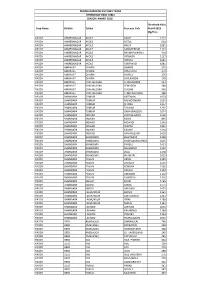
Crop Name District Taluka Revenue Cicle Threshold Yield Kharif 2018
PRADHANMANTRI PIK VIMA YOJNA THRESHOLD YIELD TABLE SEASON KHARIF 2018 Threshold Yield Crop Name District Taluka Revenue Cicle Kharif 2018 (Kg/Ha.) PADDY AHMEDNAGAR AKOLE AKOLE 1117 PADDY AHMEDNAGAR AKOLE KOTUL 994 PADDY AHMEDNAGAR AKOLE RAJUR 1081 PADDY AHMEDNAGAR AKOLE SAMSHERPUR 1117 PADDY AHMEDNAGAR AKOLE BRAMHANWADA 994 PADDY AHMEDNAGAR AKOLE VIRGAON 1117 PADDY AHMEDNAGAR AKOLE SHENDI 1081 PADDY AHMEDNAGAR AKOLE SAKIRWADI 1081 PADDY AMRAVATI DHARNI DHARNI 276 PADDY AMRAVATI DHARNI DHULGHAT 270 PADDY AMRAVATI DHARNI HARSUL 276 PADDY AMRAVATI DHARNI SAVLIKHEDA 270 PADDY AMRAVATI CHIKHALDARA CHIKHALDARA 333 PADDY AMRAVATI CHIKHALDARA SEMADOH 348 PADDY AMRAVATI CHIKHALDARA CHURNI 348 PADDY AMRAVATI CHIKHALDARA TEMBHUR SODA 348 PADDY BHANDARA TUMSAR MITEWANI 1074 PADDY BHANDARA TUMSAR NAKADONGARI 1004 PADDY BHANDARA TUMSAR SIHORA 1157 PADDY BHANDARA TUMSAR TUMSAR 1292 PADDY BHANDARA TUMSAR GARA (BAGEDA) 1074 PADDY BHANDARA MOHADI ANDHALGAON 1410 PADDY BHANDARA MOHADI KARDI 895 PADDY BHANDARA MOHADI MOHADI 1246 PADDY BHANDARA MOHADI WARTHI 1341 PADDY BHANDARA MOHADI KADARI 1410 PADDY BHANDARA MOHADI KANHALGAON 1410 PADDY BHANDARA BHANDARA BHANDARA 1238 PADDY BHANDARA BHANDARA DHARGAON (GHARGAON) 1015 PADDY BHANDARA BHANDARA PAHELA 1413 PADDY BHANDARA BHANDARA SHAHAPUR 1422 PADDY BHANDARA BHANDARA BELA 1238 PADDY BHANDARA BHANDARA KHAMARI 1015 PADDY BHANDARA PAVANI ADYAL 1295 PADDY BHANDARA PAVANI ASGAON 1405 PADDY BHANDARA PAVANI KONDHA 1326 PADDY BHANDARA PAVANI PAVANI 1102 PADDY BHANDARA PAVANI AMGAON 1102 PADDY BHANDARA PAVANI CHINCHAL 1326 PADDY BHANDARA SAKOLI EKODI 1466 PADDY BHANDARA SAKOLI SAKOLI 1475 PADDY BHANDARA SAKOLI SANGADI 1475 PADDY BHANDARA LAKHUNDUR BARVA 1161 PADDY BHANDARA LAKHUNDUR LAKHUNDUR 1261 PADDY BHANDARA LAKHUNDUR VIRALI 1169 PADDY BHANDARA LAKHUNDUR MASAL 1161 PADDY BHANDARA LAKHANI LAKHANI 1016 PADDY BHANDARA LAKHANI PALANDUR 1184 PADDY BHANDARA LAKHANI POHARA 1154 PADDY BHANDARA LAKHANI PIMPALGAON 1016 PADDY BEED AMBAJOGAI AMBAJOGAI 245 PADDY BEED AMBAJOGAI BARDAPUR 238 PADDY BEED AMBAJOGAI GHATNANDUR 238 PADDY BEED AMBAJOGAI LO. -
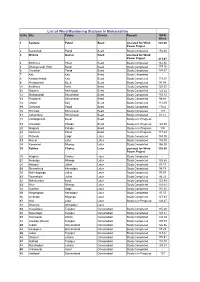
WRA List of Wind Monitoring Stations.Pdf
List of Wind Monitoring Stations In Maharashtra Sr.No Site Taluka District Remark WPD W/m2 1 Sautada Patod Beed declared for Wind 223.00 Power Project 2 Bedarwadi Patod Beed Study Completed 152.00 3 Mirkala Gaorai Beed declared for Wind Power Project 211.07 4 Sonhivra Parali Beed Study Completed 142.46 5 Dhangarwadi (Pali) Beed Beed Study Completed 179.16 6 Chumbali Patod Beed Study Completed 138.67 7 Kaij Kaij Beed Study Completed - 8 Kordyachiwadi Kaij Beed Study Completed 119.25 9 Hivrapahadi Beed Beed Study Completed 91.94 10 Ambhora Ashti Beed Study Completed 128.03 11 Nirpana Ambejogai Beed Study Completed 122.22 12 Shingarwadi Shirurkasar Beed Study Completed 103.12 13 Pimpalner Shirurkasar Beed Study Completed 99.68 14 Undari Kaij Beed Study Completed 113.45 15 Chincholi Patod Beed Study Completed 178.6 16 Bhirwadi Shirurkasar Beed Study Completed 127 17 Jatnandure Shirurkasar Beed Study Completed 87.57 18 Limbaganesh Beed Beed Study is in Progress 19 Chumbali Patoda Beed Study is in Progress 167.95 20 Waghira Patoda Beed Study is in Progress 195 21 Sonhivra Parali Beed Study is in Progress 117.29 22 Pirtanda Udgir Latur Study Completed 154.00 23 Murud Latur Latur Study Completed 143.00 24 Kasarsirsi Nilanga Latur Study Completed 156.00 25 Rohina Chakur Latur declared for Wind 226.00 Power Project 26 Wagholi Chakur Latur Study Completed - 27 Ambulga Nilanga Latur Study Completed 133.68 28 Mangrul Jalkot Latur Study Completed 85.57 29 Shivankhed Ahemdpur Latur Study Completed 94.78 30 Mali Hipparga Jalkot Latur Study Completed -

State Bank of India Lead Bank Office,Parbhani List of Allocation of Villages to Bank/Branches
STATE BANK OF INDIA LEAD BANK OFFICE,PARBHANI LIST OF ALLOCATION OF VILLAGES TO BANK/BRANCHES SUB- TOWN _ NAME OF THE Sr No Sr.No. STATE DISTRICT WARD EB LEVEL NAME TRU No_HH TOT_P Bank Branch DISTT VILL BLOCK 1 1 27 17 5 0 0 VILLAGE Simurgavhan Pathri Rural 225 1155 State Bank Of India Pathri 2 2 27 17 5 0 0 VILLAGE Zari Pathri Rural 331 1662 State Bank Of India Pathri 3 3 27 17 5 0 0 VILLAGE Warkheda Pathri Rural 265 1440 Maharashtra Gramin Bank Pathri 4 4 27 17 5 0 0 VILLAGE Jawala Zute Pathri Rural 256 1305 Maharashtra Gramin Bank Pathri 5 5 27 17 5 0 0 VILLAGE Manjarath Pathri Rural 135 685 Maharashtra Gramin Bank Pathri 6 6 27 17 5 0 0 VILLAGE Banegaon Pathri Rural 188 1043 Maharashtra Gramin Bank Pathri 7 7 27 17 5 0 0 VILLAGE Mardasgaon Pathri Rural 307 1354 HDFC BANK Sailu 8 8 27 17 5 0 0 VILLAGE Bandar Wada Pathri Rural 241 1205 State Bank Of India Pathri 9 9 27 17 5 0 0 VILLAGE Pohe Takli Pathri Rural 272 1414 State Bank Of India Pathri 10 10 27 17 5 0 0 VILLAGE Babultar Pathri Rural 311 1542 State Bank Of India Pathri 11 11 27 17 5 0 0 VILLAGE Jaitapur Wadi Pathri Rural 125 631 State Bank Of India Pathri 12 12 27 17 5 0 0 VILLAGE Tura Pathri Rural 268 1215 State Bank Of India Pathri 13 13 27 17 5 0 0 VILLAGE Takalgavhan Tanda (N.V.) Pathri Rural 96 606 Bank Of Maharashtra Pathri 14 14 27 17 5 0 0 VILLAGE Takalgavhan Parbhani Rural 161 709 Central Bank Of India Parbhani 15 15 27 17 5 0 0 VILLAGE Andhapuri Pathri Rural 188 1004 Bank Of Maharashtra Pathri 16 16 27 17 5 0 0 VILLAGE Kansoor Tanda (N.V.) Pathri Rural 64 381 Bank -

Sangram Kendra
Sangram Kendra District Taluka Village VLE Name Akola Akola AGAR PRAMOD R D Akola Akola AKOLA N KASHIRAM A Akola Akola AKOLA JP Shriram Mahajan Akola Akola AKOLA NW RP Vishal Shyam Pandey Akola Akola AKOLA NW RP-AC1 Vishal Shyam Pandey Akola Akola AKOLA OPP CO Dhammapal Mukundrao Umale Akola Akola AKOLA OPP CO-AC1 Dhammapal Mukundrao Umale Akola Akola AKOLA RP Rahul Rameshrao Deshmukh Akola Akola ANVI 2 Ujwala Shriram Khandare Akola Akola APATAPA Meena Himmat Deshmukh Akola Akola BABHULGAON A Jagdish Maroti Malthane Akola Akola BHAURAD MR Jagdish Gulabrao Deshmukh Akola Akola BORGAON M2 Amol Madhukar Ingale Akola Akola BORGAON MANJU N NARAYANRAO A Akola Akola DAHIHANDA RAJESH C T Akola Akola GANDHIGRAM Nilesh Ramesh Shirsat Akola Akola GOREGAON KD 2 Sandip Ramrao Mapari Akola Akola KANSHIVANI Pravin Nagorao Kshirsagar Akola Akola KASALI KHURD Kailash Shankar Shirsat Akola Akola KAULKHED RD DK Jyoti Amol Ambuskar Akola Akola KHADKI BU Kundan Ratangir Gosavi Akola Akola KHARAP BK Ishwar Bhujendra Bhati Akola Akola KOLAMBHI Amol Balabhau Badhe Akola Akola KURANKHED Sanjeevani Deshmukh Akola Akola MAJALAPUR Abdul Anis Abdul Shahid Akola Akola MALKAPUR V RAMRAO G Akola Akola MAZOD Sahebrao Ramkrushna Khandare Akola Akola MHAISANG Bhushan Chandrashekhar Gawande Akola Akola MHATODI Harish Dinkar Bhande Akola Akola MORGAON BHAK Gopal Shrikrishna Bhakare Akola Akola MOTHI UMRI A BHIMRAO KAPAL Akola Akola PALSO Siddheshwar Narayan Gawande Akola Akola PATUR NANDAPUR Atul Ramesh Ayachit Akola Akola RANPISE NAGAR Shubhangi Rajnish Thakare Akola Akola -

Maharashtra State Council of Examinations, Pune
MAHARASHTRA STATE COUNCIL OF EXAMINATIONS, PUNE NATIONAL MEANS CUM MERIT SCHOLARSHIP SCHEME EXAM 2014-15 ( STD - 8 th ) CENTRE WISE LIST OF SCHOOL DISTRICT: 54 PARBHANI Date : 04/12/2014 CENTRE : 5403 SAVITRIBAI PHULE (GIRLS) MADHYAMIK VIDYALAYA, PARBHANI Page : 1 of 2 GENERAL MENTAL ABILITY TEXT SCHOLASTIC APTITUDE TEST SCHOOL CODE TOTAL & NAME MAR URD HIN GUJ ENG SIN TEL KAN MAR URD HIN GUJ ENG SIN TEL KAN CAND. G-1 / G-15 G-2 / G-25 G-3 / G-35 G-4 / G-45 G-5 G-6 / G-65 G-7 / G-75 G-8 / G-85 S-1 / S-15 S-2 / S-25 S-3 / S-35 S-4 / S-45 S-5 S-6 / S-65 S-7 / S-75 S-8 / S-85 5402004 - VIDYAPRASARINI H.S PURNA DIST 0 11 0 0 0 0 0 0 0 0 0 0 0 0 0 0 11 0 0 0 0 0 0 0 0 0 0 0 0 0 11 PARBHANI ANAND NAGAR PURNA 5402025 - VIVEKANAND VIDY. KATNESHWAR 3 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 0 0 0 0 0 0 0 0 0 0 0 0 0 0 3 VIVEKANAND VIDY. KATNESHWAR 5402027 - SOU.RUKHMINIBAI AMBORE 26 0 0 0 0 0 0 0 0 0 0 0 0 0 0 26 0 0 0 0 0 0 0 0 0 0 0 0 0 0 26 VIDYALAY MIRKHEL(STN) AT RAMAPUR PO MIRKHEL TA 5403008 - JAWAHAR VID JINTUR JAWAHAR 34 0 0 0 0 0 0 0 0 0 0 0 0 0 0 34 0 0 0 0 0 0 0 0 0 0 0 0 0 0 34 COLONY KHAIRI PLOT JINTUR 5403010 - SMT.SHKUNTALABAI BORDIKAR GIRLS 0 12 0 0 0 0 0 0 0 0 0 0 0 0 0 0 12 0 0 0 0 0 0 0 0 0 0 0 0 0 12 VID JINTUR JUNI MUNSFFI JINTUR 5403013 - SAIBABA VID. -

Village Map Jintur Taluka: Parbhani District: Parbhani Pingli Kothala Jodparli Zari
Aundha (Nagnath) Sailu Village Map Jintur Taluka: Parbhani District: Parbhani Pingli kothala Jodparli Zari Takli Bobade Mirzapur Digras Mandwa Kumbhari Khanapur Tarf Zari Kashtagaon Karla Sadegaon Gokulwadi Sawangi kh. Wadgaon tarf takli GovindpurSarangapur Jalalpur Pimpalgaon sayyadmia Nandapur Ekrukha Tarf Pedgaon Sultanpur Wadi damai µ Ismailpur tarf parbhani Arvi 3.5 1.75 0 3.5 7 10.5 Basmath Hingla Sambar km Sanpuri Dharangaon Kinhola Matakarala Takli kumbhakarna Mangangaon Bramhapuri tarf pedgaon Narsapur tarf pedgaon Sukapurwadi Karadgaon Shahapur Pimpalgaon tong Tuljapur Pedgaon Satla Durdi Samsapur Location Index Dhar Nandkheda Deothana Murumba Panhera Hasnapur Dharmapuri Bhogaon District Index Saba Nandurbar Mohapuri Nandgaon kh. Bhandara Wangi Nandgaon bk. Dhule Amravati Nagpur Gondiya Jalgaon Alapur pandhari Akola Wardha Parawa Buldana Aland Nashik Washim Chandrapur Jamb Parbhani (M Cl) Rahati Yavatmal Aurangabad Kaudgaon tarf singanapur Asola Palghar Manwath PARBHANI Jalna Hingoli Gadchiroli !( Thane !. Ahmednagar Parbhani Dhangarwadi Mumbai Suburban Nanded Karegaon Mumbai Bid Ukhalad Raigarh Pune Latur Bidar Mandakhali Osmanabad Bramhangaon Sonna Babhali Satara Solapur Raipur Bramhapuri tarf lohgaon Ratnagiri Sangli Nagapur Pimpari deshmukh Maharashtra State Sendra Balsa kh. Pingali Kolhapur Dafwadi Babhulgaon Narsapur tarf parbhani Tattu jawala Ujalamba Sindhudurg Taroda Dharwad Sayala Borwand kh. Ithalapur Deshmukh Umari Taluka Index Takalgavhan Mirkhel Pimpalgaon Thombare Paralgavhan Tadlimbla Singnapur Jintur Anandwadi Borwand bk. Pandhari Surpimpari Purna Amadapur Varpud Zadgaon Purjawala Sailu Lohagaon Pathra Tamaswadi Wadgaon tarf bharaswad Tadpangari Sahajpur Jawala Manwath Parbhani Bharaswad Kuotamwadi Pathri Pegargavhan Sirsi kh. Purna Pokharni Ambe takli Dampuri Sirsi bk. Legend Sonpeth Palam Kailaswadi Indewadi !( Taluka Head Quarter Gangakhed Bramhapuri tarf pathri Porwad Thola District Head Quarter !. District: Parbhani Daithana Railway Dhondi Malsonna Salapuri National Highway Village maps from Land Record Department, GoM. -

Pincode Officename Mumbai G.P.O. Bazargate S.O M.P.T. S.O Stock
pincode officename districtname statename 400001 Mumbai G.P.O. Mumbai MAHARASHTRA 400001 Bazargate S.O Mumbai MAHARASHTRA 400001 M.P.T. S.O Mumbai MAHARASHTRA 400001 Stock Exchange S.O Mumbai MAHARASHTRA 400001 Tajmahal S.O Mumbai MAHARASHTRA 400001 Town Hall S.O (Mumbai) Mumbai MAHARASHTRA 400002 Kalbadevi H.O Mumbai MAHARASHTRA 400002 S. C. Court S.O Mumbai MAHARASHTRA 400002 Thakurdwar S.O Mumbai MAHARASHTRA 400003 B.P.Lane S.O Mumbai MAHARASHTRA 400003 Mandvi S.O (Mumbai) Mumbai MAHARASHTRA 400003 Masjid S.O Mumbai MAHARASHTRA 400003 Null Bazar S.O Mumbai MAHARASHTRA 400004 Ambewadi S.O (Mumbai) Mumbai MAHARASHTRA 400004 Charni Road S.O Mumbai MAHARASHTRA 400004 Chaupati S.O Mumbai MAHARASHTRA 400004 Girgaon S.O Mumbai MAHARASHTRA 400004 Madhavbaug S.O Mumbai MAHARASHTRA 400004 Opera House S.O Mumbai MAHARASHTRA 400005 Colaba Bazar S.O Mumbai MAHARASHTRA 400005 Asvini S.O Mumbai MAHARASHTRA 400005 Colaba S.O Mumbai MAHARASHTRA 400005 Holiday Camp S.O Mumbai MAHARASHTRA 400005 V.W.T.C. S.O Mumbai MAHARASHTRA 400006 Malabar Hill S.O Mumbai MAHARASHTRA 400007 Bharat Nagar S.O (Mumbai) Mumbai MAHARASHTRA 400007 S V Marg S.O Mumbai MAHARASHTRA 400007 Grant Road S.O Mumbai MAHARASHTRA 400007 N.S.Patkar Marg S.O Mumbai MAHARASHTRA 400007 Tardeo S.O Mumbai MAHARASHTRA 400008 Mumbai Central H.O Mumbai MAHARASHTRA 400008 J.J.Hospital S.O Mumbai MAHARASHTRA 400008 Kamathipura S.O Mumbai MAHARASHTRA 400008 Falkland Road S.O Mumbai MAHARASHTRA 400008 M A Marg S.O Mumbai MAHARASHTRA 400009 Noor Baug S.O Mumbai MAHARASHTRA 400009 Chinchbunder S.O