J Gericke Orcid.Org/ 0000-0003-0038-3374
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna Et Al
US 2016O174603A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna et al. (43) Pub. Date: Jun. 23, 2016 (54) ELECTRONIC VAPORLIQUID (52) U.S. Cl. COMPOSITION AND METHOD OF USE CPC ................. A24B 15/16 (2013.01); A24B 15/18 (2013.01); A24F 47/002 (2013.01) (71) Applicants: Sahan Abayarathna, Missouri City, TX 57 ABSTRACT (US); Michael Jaehne, Missouri CIty, An(57) e-liquid for use in electronic cigarettes which utilizes- a TX (US) vaporizing base (either propylene glycol, vegetable glycerin, (72) Inventors: Sahan Abayarathna, MissOU1 City,- 0 TX generallyor mixture at of a 0.001 the two) g-2.0 mixed g per with 1 mL an ratio. herbal The powder herbal extract TX(US); (US) Michael Jaehne, Missouri CIty, can be any of the following:- - - Kanna (Sceletium tortuosum), Blue lotus (Nymphaea caerulea), Salvia (Salvia divinorum), Salvia eivinorm, Kratom (Mitragyna speciosa), Celandine (21) Appl. No.: 14/581,179 poppy (Stylophorum diphyllum), Mugwort (Artemisia), Coltsfoot leaf (Tussilago farfara), California poppy (Eschscholzia Californica), Sinicuichi (Heimia Salicifolia), (22) Filed: Dec. 23, 2014 St. John's Wort (Hypericum perforatum), Yerba lenna yesca A rtemisia scoparia), CaleaCal Zacatechichihichi (Calea(Cal termifolia), Leonurus Sibericus (Leonurus Sibiricus), Wild dagga (Leono Publication Classification tis leonurus), Klip dagga (Leonotis nepetifolia), Damiana (Turnera diffiisa), Kava (Piper methysticum), Scotch broom (51) Int. Cl. tops (Cytisus scoparius), Valarien (Valeriana officinalis), A24B 15/16 (2006.01) Indian warrior (Pedicularis densiflora), Wild lettuce (Lactuca A24F 47/00 (2006.01) virosa), Skullcap (Scutellaria lateriflora), Red Clover (Trifo A24B I5/8 (2006.01) lium pretense), and/or combinations therein. -

Investigation Into Potential Endocrine Disruptive Effects of Sceletium Tortuosum
Investigation into potential endocrine disruptive effects of Sceletium tortuosum by Letitia Louw Dissertation presented for the degree of Master of Science in the Faculty of Science at Stellenbosch University Supervisor: Prof C. Smith Co-supervisor: Prof AC. Swart March 2018 Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. March 2018 Copyright © 2018 Stellenbosch University All rights reserved i Stellenbosch University https://scholar.sun.ac.za What if I fall? Oh, but darling what if you fly? ii Stellenbosch University https://scholar.sun.ac.za ABSTRACT Depression has been recognised by the World Health Organisation (WHO) as the leading cause of disability, affecting an estimated 300 million people globally. To date antidepressants are prescribed as the first step in the treatment strategy. However, finding the appropriate antidepressant is often a lengthy process and is usually accompanied by side effects. A major and often unexpected side effect is reduced sexual function, which has been reported to aggravate depression and could possibly lead to poor compliance to medication. Sceletium tortuosum is a native South African plant, which has exhibited both antidepressant and anxiolytic properties. Although the exact mechanism of action remains to be elucidated, there are currently two hypotheses which attempt to explain it’s mechanism of action. -

Natural Products of Relevance in the Prevention and Supportive Treatment of Depression
Psychiatr. Pol. 2015; 49(3): 435–453 PL ISSN 0033-2674 (PRINT), ISSN 2391-5854 (ONLINE) www.psychiatriapolska.pl DOI: http://dx.doi.org/10.12740/PP/29367 Natural products of relevance in the prevention and supportive treatment of depression Bożena Muszyńska1, Maciej Łojewski 1,Jacek Rojowski 2, Włodzimierz Opoka 2, Katarzyna Sułkowska-Ziaja1 1Chair and Department of Pharmaceutical Botany, Jagiellonian University Medical College Head: prof. dr hab. H. Ekiert 2Chair of Inorganic and Analytical Chemistry, Faculty of Pharmacy, Jagiellonian University Medical College Head: dr hab. W. Opoka, prof. of Jagiellonian University Summary The use of herbs or their parts: leaves, roots, rhizomes, flowers, seeds, natural strains, as well as extracts or isolated metabolites is becoming more and more popular. Natural remedies not only act prophylactically, but also help to alleviate symptoms of many diseases and enhance the overall functioning of the internal organs. Many raw materials of natural origin plays a role in treatment of health problems, and also in case of serious diseases such as depression. Depres- sion (affective disorder) now affects about 10% of the population, but in next few years due to the development of civilization and increasing pace of life, the probable number of people suffering from this disease can grow rapidly. Natural raw materials such as Bacopa monnieri, Crocus sativus, Eleutherococcus senticosus, Griffonia simplicifolia, Hypericum perforatum, Sceletium tortuosum, Piper methysticum, Rhodiola rosea, Aspalathus linearis, Camellia sinensis, Ficus carica, Lycium chinense, Cuminum cyminum, Panax Ginseng can effectively assist the prevention and treatment of depression. Daily diet may also have positive effect in prevention of this disease. -

Plant List 2021-06-24
Plant List 2021-10-02 (08:28) Plant Plant Name Botanical Name in Price Stock Per Unit AFRICAN DREAM ROOT - 1 Silene capensis Yes R92 AFRICAN DREAM ROOT - 2 Silene undulata Yes R92 AFRICAN POTATO Hypoxis hemerocallidea Yes R89 AFRICAN POTATO - SILVER-LEAFED STAR FLOWER Hypoxis rigidula Yes R89 AGASTACHE - GOLDEN JUBILEE Agastache foeniculum No R52 AGASTACHE - HYSSOP, WRINKLED GIANT HYSSOP Agastache rugosa Yes R59 AGASTACHE - LICORICE MINT HYSSOP Agastache rupestris No R59 AGASTACHE - PINK POP Agastache astromontana No R54 AGRIMONY Agrimonia eupatoria No R54 AJWAIN Trachyspermum ammi No R49 ALFALFA Medicago sativa Yes R59 ALOE VERA - ORANGE FLOWER A. barbadensis Yes R59 ALOE VERA - YELLOW FLOWER syn A. barbadensis 'Miller' No R59 AMARANTH - ‘LOVE-LIES-BLEEDING’ Amaranthus caudatus No R49 AMARANTH - CHINESE SPINACH Amaranthus species No R49 AMARANTH - GOLDEN GIANT Amaranthus cruentas No R49 AMARANTH - RED LEAF Amaranthus cruentas No R49 ARTICHOKE - GREEN GLOBE Cynara scolymus Yes R54 ARTICHOKE - JERUSALEM Helianthus tuberosus Yes R64 ARTICHOKE - PURPLE GLOBE Cynara scolymus No R54 ASHWAGANDA, INDIAN GINSENG Withania somniferia Yes R59 ASPARAGUS - GARDEN Asparagus officinalis Yes R54 BALLOON FLOWER - PURPLE Platycodon grandiflorus 'Apoyama' Yes R59 BALLOON FLOWER - WHITE Platycodon grandiflorus var. Albus No R59 BASIL - CAMPHOR Ocimum kilimandscharicum Yes R59 BASIL HOLY - GREEN TULSI, RAM TULSI Ocimum Sanctum Yes R54 BASIL HOLY - TULSI KAPOOR Ocimum sanctum Linn. No R54 BASIL HOLY - TULSI TEMPERATE Ocimum africanum No R54 BASIL HOLY - TULSI -

Medicinal Plants Used in the Treatment of Human Immunodeficiency Virus
International Journal of Molecular Sciences Review Medicinal Plants Used in the Treatment of Human Immunodeficiency Virus Bahare Salehi 1,2 ID , Nanjangud V. Anil Kumar 3 ID , Bilge ¸Sener 4, Mehdi Sharifi-Rad 5,*, Mehtap Kılıç 4, Gail B. Mahady 6, Sanja Vlaisavljevic 7, Marcello Iriti 8,* ID , Farzad Kobarfard 9,10, William N. Setzer 11,*, Seyed Abdulmajid Ayatollahi 9,12,13, Athar Ata 13 and Javad Sharifi-Rad 9,13,* ID 1 Medical Ethics and Law Research Center, Shahid Beheshti University of Medical Sciences, 88777539 Tehran, Iran; [email protected] 2 Student Research Committee, Shahid Beheshti University of Medical Sciences, 22439789 Tehran, Iran 3 Department of Chemistry, Manipal Institute of Technology, Manipal University, Manipal 576104, India; [email protected] 4 Department of Pharmacognosy, Gazi University, Faculty of Pharmacy, 06330 Ankara, Turkey; [email protected] (B.¸S.);[email protected] (M.K.) 5 Department of Medical Parasitology, Zabol University of Medical Sciences, 61663-335 Zabol, Iran 6 PAHO/WHO Collaborating Centre for Traditional Medicine, College of Pharmacy, University of Illinois, 833 S. Wood St., Chicago, IL 60612, USA; [email protected] 7 Department of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Trg Dositeja Obradovica 3, 21000 Novi Sad, Serbia; [email protected] 8 Department of Agricultural and Environmental Sciences, Milan State University, 20133 Milan, Italy 9 Phytochemistry Research Center, Shahid Beheshti University of -

The Importance of Sceletium Tortuosum (L.) N.E. Brown and Its
Chapter The Importance of Sceletium tortuosum (L.) N.E. Brown and Its Viability as a Traditional African Medicinal Plant Richard James Faber, Charles Petrus Laubscher and Muhali Olaide Jimoh Abstract Sceletium tortuosum is a succulent plant that belongs to the family Mesembryanthemaceae (Aizoaceae). It is indigenous to South Africa, where it is well known by the indigenous people, especially in Namaqualand where the plant is utilized regularly for its medicinal and psycho-active properties. The main alkaloids responsible for these properties are mesembrine, mesembrenine (mesembrenone), and mesembrenol. The potential of the plant to be an alternative supplement in the promotion of health and treating a variety of psychological and psychiatric disor- ders such as depression and anxiety has stimulated interest in its pharmacological property and possibility of its commercialization. The economic value of indig- enous medicinal plants in South Africa is approximately US$60 000 000 or R4 000 000 000 annually. Thus, interest in the knowledge and use of Traditional African Medicinal Plants (TAMP) as well as meeting pharmacological and economic needs of ever-increasing human population has led to the commercialization of traditional African medicines at a fast rate. It was found that S. tortuosum has clear pharmaceutical and economical importance and is one of the only known plants to contain the alkaloids mesembrenone and mesembrine which can be utilized for the promotion of health and/or treating a variety of psychological disorders such as anxiety and depression. Keywords: African medicine, Aizoaceae, alkaloids, hydroponics, mesembrine, mesembrenine, mesembrenol, mesembryanthemaceae 1. Introduction Sceletium tortuosum (L.) N.E. Br. and Sceletium expansum L. -

Diversity of Endophytic Fungi Possessing Bioactive Compounds Isolated from Selected Medicinal Plants
Diversity of endophytic fungi possessing bioactive compounds isolated from selected medicinal plants Madira Coutlyne Manganyi C9orcid.org/0000-0002-0209-5547 Dissertation submitted in fulfilment of the requirements for the degree Doctor of Philosophy in Biology (Molecular Microbiology) at the Mafikeng Campus of the North-West University Promoter: Professor CN A TEBA Co-promoters: Professor T REGNIER (TUT) Professor CC BEZUIDENHOUT (NWU) Graduation October 2018 Student number: 26853795 http://dspace.nwu.ac.za/ DECLARATION I, Madira Coutlyne Manganyi, declare that the thesis entitled "Diversity of endophytic fungi possessing bioactive compounds isolated from selected medicinal plants", hereby submitted for the degree of Doctor of Science in Biology (Molecular Microbiology) , has not previously been submitted by me for a degree at this or any other university. I further declare that this is my work in design and execution and that all materials contained herein have been duly acknowledged . Signed ... ......... ... ...... ...... ......... this the ... ........ ... ....... .... day of ...... ... ..... .... 2017 Signature:...... .... ..... ............................... Date : .. ... ......... ... ...... ......... ......... ...... MC Manganyi (Student) Signature :... .. ............... ......... ....... .. ..... .... Date : ...... ... ....... ...... ... ........... ........ .. .. Prof CN Ateba (Supervisor) Signature :............... .............................. .. Date : .... .. ............ .. .. ..... ... ............... .. Prof T Regnier -

Mood+ Technical Data Sheet
All-Natural Mood Support* TECHNICAL DATA *These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. The Mental Wellness Company Mood+ Technical Data Sheet A comprehensive blend of research-backed premium herbs for mood support. Reduces tension and nervousness; Improves disposition and overall well-being.* KEY INGREDIENTS Zembrin® (Sceletium tortuosum) - also known as Kanna, Sceletium tortuosum was traditionally used by the San and Khoi peoples of Southern Africa as an analgesic (pain reliever), sedative, tonic (energy/stamina) and mood elevator. The traditionally prepared dried plant material is chewed, smoked, or powdered and inhaled as a snuff. It is also used as a tea or tincture. It was typically used in cognitively stressing situations such as hunting or coping in which its “adaptogenic” (stress-balancing) properties are readily apparent. Lower daily doses are known to have a subtle effect providing a sense of serenity and at the same time an elevated sense of alertness and awareness, while larger doses lead to a transient euphoria. Zembrin delivers a wide range of positive health benefits, including elevated mood and mental clarity; improved focus and memory; increased energy and motivation; lower stress hormone levels; and decreased everyday anxiety. Kanna is known to influence the amygdala of the brain (a brain region central in emotional processing) and is known to also have inhibitory effects on both the serotonin transporter as well as an enzyme known as phosphodiesterase 4 (PDE4); both of these proteins existing in the amygdala. Kanna contains a family of alkaloids (mesembrine, mesembrenone, mesembrenol, and mesembranol) confirmed to have dual effects on inhibiting serotonin reuptake and PDE4. -

Psychoactive Natural Products: Overview of Recent Developments
12 Ann Ist Super Sanità 2014 | Vol. 50, No. 1: 12-27 DOI: 10.4415/ANN_14_01_04 Psychoactive natural products: overview of recent developments István Ujváry REVIEWS iKem BT, Budapest, Hungary AND Abstract Natural psychoactive substances have fascinated the curious mind of shamans, artists, Key words ARTICLES scholars and laymen since antiquity. During the twentieth century, the chemical com- • ethnopharmacology position of the most important psychoactive drugs, that is opium, cannabis, coca and • mode of action “magic mushrooms”, has been fully elucidated. The mode of action of the principal in- • natural products gredients has also been deciphered at the molecular level. In the past two decades, the • psychopharmacology RIGINAL use of herbal drugs, such as kava, kratom and Salvia divinorum, began to spread beyond • toxicology O their traditional geographical and cultural boundaries. The aim of the present paper is to briefly summarize recent findings on the psychopharmacology of the most prominent psychoactive natural products. Current knowledge on a few lesser-known drugs, includ- ing bufotenine, glaucine, kava, betel, pituri, lettuce opium and kanna is also reviewed. In addition, selected cases of alleged natural (or semi-natural) products are also mentioned. O, mickle is the powerful grace that lies In herbs, plants, stones, and their true qualities William Shakespeare (Romeo and Juliet) INTRODUCTION Historical background of psychoactive natural During the past 200 years, there has been major pro- products research gress in our understanding of the composition and ef- The biochemical machinery of an organism generates fects of many psychoactive natural products, particular- many structurally related chemicals (Nature’s “combinato- ly those that have therapeutic uses. -
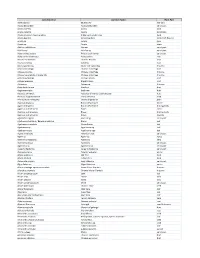
Testing Services List V2020
Latin Binomial Common Name Plant Part Abies sibirica Siberian Fir leaf (oil) Acacia Berlandieri Acacia Berlandieri aerial part Acacia catechu Acacia bark Acacia catechu Acacia gum/resin Acacia nilotica / Acacia arabica Indian gum arabic tree bark Acacia Rigidula Acacia Rigidula herb (leaf, flower) Acacia sp. Acacia gum Acacia sp. Acacia stem Achillea millefolium Yarrow aerial part Achillea sp. Achillea sp. aerial part Achyranthes aspera Prickly chaff flower aerial part Achyranthes bidentata Achyranthes root Aconite carmichaeli Chinese Aconite root Acorus calamus Calamus root Acorus gramineus Grass-leaf sweetflag rhizome Actaea cimicifuga Chinese cimicifuga root Actaea dahurica Chinese cimicifuga rhizome Actaea heracleifolia / Sheng Ma Chinese cimicifuga rhizome Actaea podocarpa Yellow Cohosh root Actaea racemosa Black Cohosh root Actaea sp. Actaea sp. rhizome Actinidia deliciosa Kiwifruit fruit Aegle marmelos Bael tree fruit Aesculus chinensis Aesculus chinensis [Sapindaceae] fruit Aesculus hippocastanum Horse chestnut seed Aframomum melegueta Grains of paradise grain Agaricus bisporus Button Mushroom entire Agaricus bisporus Button Mushroom fruiting body Agaricus subrufescens Blazei entire Agaricus subrufescens Blazei fruiting body Agaricus subrufescens Blazei mycelia Agastache rugosa Huo Xiang aerial part Agathosma betulina / Barosma betulina Buchu leaf Agathosma crenulata Ovate Buchu leaf Agathosma sp. Agathosma sp. leaf Agathosma spp. Agathosma spp. leaf Agave americana American aloe aerial part Agave sp. Agave sp. syrup Agrimonia -

Exploring Standardized Zembrin® Extracts from the South African
Internationa Journal of Complementary & Alternative Medicine Exploring Standardized Zembrin® Extracts from the South African plant Sceletium tortuosum in Dual Targeting Phosphodiesterase-4 (PDE-4) and Serotonin Reuptake Inhibition as potential treatment in Schizophrenia Abstract Mini Review Converging evidence suggests that Disrupted in Schizophrenia (DISC-1) is a (Phosphodiesterase Subtype-4) plays a crucial role in neurodevelopment and SimonVolume 6Chiu Issue1,4 5*, - Hana2017 Raheb1, Kristen neuro-inflammationrecognized risk gene in for schizophrenia. schizophrenia. Serotonin The interaction has drawn of increasedDISC- with attention PDE-4 Terpstra1,5, Josh Vaughan1,6, Autumn for its role in modulating cognition function. Hence dual targeting Selective Carrie1,7, Michel Farina-Woodbury2, Yves 1,8 1,4 1 towards augmenting antipsychotics in schizophrenia. Translational studies show Bureau , Zack Cernovsky , Jirui Hou , 3 8 thatSerotonin the family Reuptake of mesembrane-related site (SSRI) and PDE-4 alkaloids may offer isolated novel from therapeutic the South paradigm African John Copen , Mariwan Husni , Vladimer Badmeav9, Mujeeb Shad10, Zach Suntras8 4 allosteric site. The promising findings warrant a proof-of-concept randomized and Nigel Gericke11 controlledplant: Sceletium study Zembrintortuosum extract have inbeen schizophrenia. shown to hit dual targets: SSRI and PDE- 1Lawson Health Research Institute, Canada 2Department of Psychiatry, University of Puerto Rico, USA Keywords: Schizophrenia; DISC-1 gene; Serotonin; Phosphodiesterase; -

Herbal Drugs of Abuse Glaucium Flavum and Sceletium Tortuosum: Metabolism and Toxicological Detectability of Their Alkaloids Gl
Herbal Drugs of Abuse Glaucium flavum and Sceletium tortuosum: Metabolism and toxicological detectability of their alkaloids glaucine, mesembrine and mesembrenone studied in rat urine and human liver preparations using GC-MS, LC-MS, LC-HR-MSn, and NMR Dissertation zur Erlangung des Grades des Doktors der Naturwissenschaften der Naturwissenschaftlich-Technischen Fakultät III - Chemie, Pharmazie, Bio- und Werkstoffwissenschaften der Universität des Saarlandes Von Golo Magnus Joris Onno Max Meyer Saarbrücken 2014 Tag des Kolloquiums: 21.11.2014 Dekan: Univ.-Prof. Dr.-Ing. Dirk Bähre Berichterstatter: Univ.-Prof. Dr. Dr. h.c. Hans H. Maurer Univ.-Prof. Dr. Rolf W. Hartmann Vorsitz: Univ.-Prof. Dr. Adolfo Cavalié Akad. Mitarbeiter: Dr. Josef Zapp 1 Die folgende Arbeit entstand unter der Anleitung von Herrn Professor Dr. Dr. h.c. Hans H. Maurer in der Abteilung Experimentelle und Klinische Toxikologie der Fachrichtung 2.4 Experimentelle und Klinische Pharmakologie und Toxikologie der Universität des Saarlandes in Homburg/Saar von Juni 2010 bis Januar 2014. Mein besonderer Dank gilt: Professor Hans H. Maurer für die Aufnahme in den Arbeitskreis und die damit erhaltene Möglichkeit zum Erlernen des Handwerkszeugs des wissenschaftlichen Arbeitens, das Vertrauen, den eigenen Ideen nachgehen zu können, die vielen Fachkongresse besuchen zu dürfen und der ständig offen stehenden Tür, Professor Rolf W. Hartmann für die Übernahme des Koreferats, Allen Kolleginnen und Kollegen für die schöne Zeit, Hilfe bei der Einarbeitung, insbesondere Dirk Wissenbach, Hilfe bei Versuchen, Vererbung von Fällen, Diskussionsbereitschaft, Dr. Markus R. Meyer für die ständige Unterstützung zum eigenverantwortlichen Arbeiten aber auch für die Antworten, Einschätzungen und Bewertungen bei den zu diskutierenden Themen, Armin Weber für seine permanente Hilfsbereitschaft und großes Engagement in allen fachlichen und privaten Angelegenheiten, Gabriele Ulrich für die spannenden Rattenversuche und Carsten Schröder für technische Unterstützung und den künstlerischen Support sowie Dr.