Optumrx Brand Pipeline Forecast
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optumrx Brand Pipeline Forecast
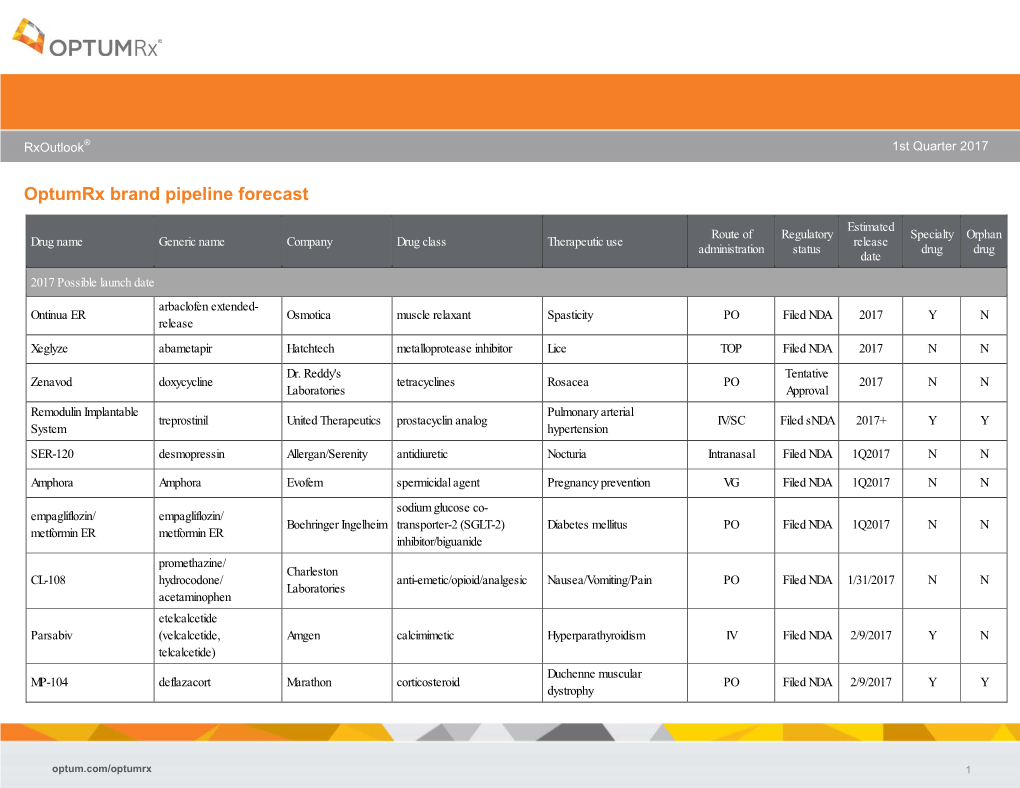
RxOutlook® 1st Quarter 2019 OptumRx brand pipeline forecast Route of Regulatory Estimated Specialty Orphan Drug name Generic name Company Drug class Therapeutic use administration status release date drug drug 2019 Possible launch date Ophthalmological DS-300 DS-300 Eton undisclosed SC Filed NDA 2019 unknown N disease anti-sclerostin Evenity romosozumab Amgen Osteoporosis SC Filed NDA 2/2019 Y N monoclonal antibody tetrahydrofolate iclaprim iclaprim Motif Bio Bacterial infections IV Filed NDA 2/13/2019 Y Y dehydrogenase inhibitor tazarotene/ IDP-118 Valeant retinoid/ corticosteroid Psoriasis TOP Filed NDA 2/15/2019 N N halobetasol adenosine deaminase Mavenclad cladribine Merck/ Teva resistant Multiple sclerosis PO Filed NDA 2/15/2019 Y N deoxyadenosine analog Lotemax Gel loteprednol Valeant corticosteroid Ocular inflammation OP Filed NDA 2/25/2019 N N Nex Gen etabonate turoctocog alfa glyco-PEGylated factor NN-7088 Novo Nordisk Hemophilia IV/SC Filed BLA 2/27/2019 Y N pegol VIII derivative selective sphingosine-1 BAF-312 siponimod Novartis phosphate receptor Multiple sclerosis PO Filed NDA 3/1/2019 Y N agonist midazolam midazolam UCB benzodiazepine Seizures Intranasal Filed NDA 3/1/2019 N Y (USL-261) XeriSol glucagon Xeris glucagon analog Diabetes mellitus SC Filed NDA 3/1/2019 N N Glucagon optum.com/optumrx 1 RxOutlook® 1st Quarter 2019 Route of Regulatory Estimated Specialty Orphan Drug name Generic name Company Drug class Therapeutic use administration status release date drug drug dopamine receptor JZP-507 sodium oxybate Jazz Narcolepsy -

Safety and Tolerability of Arimoclomol in Patients with Sporadic Inclusion Body Myositis: a Randomised, Double-Blind, Placebo-Controlled, Proof-Of-Concept Trial
Safety and tolerability of arimoclomol in patients with sporadic inclusion body myositis: a randomised, double-blind, placebo-controlled, proof-of-concept trial Sporadic inclusion body myositis (IBM) is the commonest idiopathic inflammatory myopathy (IIM) occurring in patients over the age of 50 years.1-4 The prevalence of IBM differs between different populations and ethnic groups, with estimates between 4.9-70.6 per million population.5-10 IBM is distinguished from the other IIM by early asymmetric finger flexor and knee extensor weakness (leading to loss of hand function and propensity to fall) and resistance to immunosuppressive therapy. Any skeletal muscle can be affected, including oesophageal and pharyngeal muscles. Late-stage disease can be characterized by very significant morbidity including disability and loss of quality of life. Death in IBM is related to malnutrition, cachexia, aspiration, respiratory infection and respiratory failure, as a consequence of dysphagia, severe global weakness and weakness of the respiratory muscles.1-4 In a Dutch cohort, end-of-life care interventions were used by 13% of patients with IBM, highlighting the morbidity experienced by these patients and the need of supportive and palliative care in this disease.2 The aetiopathogenesis of IBM remains uncertain. The varied pathological findings observed have driven a number of theories including viral infection, accumulation of toxic proteins, autoimmune attack, myonuclear degeneration, endoplasmic reticulum stress and impairment of autophagy and proteasomal proteolysis.11-15 Muscle biopsies from patients with IBM typically show several different pathological features broadly described as inflammatory or degenerative. Inflammatory features include endomysial infiltration by mononuclear cells (predominantly CD8+ T- cells), which surround and invade otherwise normal appearing muscle fibres, and overexpression of major histocompatibility complex (MHC) class I, which is not constitutively expressed by skeletal muscle. -

Pioneering New Markets Changing the Standard of Care
ANNUAL 2020 REPORT Pioneering New Markets Changing the Standard of Care UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 10-K ☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2020 □ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 000-19125 Ionis Pharmaceuticals, Inc. (Exact name of Registrant as specified in its charter) Delaware 33-0336973 (State or other jurisdiction of (IRS Employer incorporation or organization) Identification No.) 2855 Gazelle Court, Carlsbad, CA 92010 (Address of Principal Executive Offices) (Zip Code) 760-931-9200 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading symbol Name of each exchange on which registered Common Stock, $.001 Par Value ‘‘IONS’’ The Nasdaq Stock Market LLC Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No □ Indicate by check if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes □ No ☒ Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Horizon Scanning Status Report June 2019
Statement of Funding and Purpose This report incorporates data collected during implementation of the Patient-Centered Outcomes Research Institute (PCORI) Health Care Horizon Scanning System, operated by ECRI Institute under contract to PCORI, Washington, DC (Contract No. MSA-HORIZSCAN-ECRI-ENG- 2018.7.12). The findings and conclusions in this document are those of the authors, who are responsible for its content. No statement in this report should be construed as an official position of PCORI. An intervention that potentially meets inclusion criteria might not appear in this report simply because the horizon scanning system has not yet detected it or it does not yet meet inclusion criteria outlined in the PCORI Health Care Horizon Scanning System: Horizon Scanning Protocol and Operations Manual. Inclusion or absence of interventions in the horizon scanning reports will change over time as new information is collected; therefore, inclusion or absence should not be construed as either an endorsement or rejection of specific interventions. A representative from PCORI served as a contracting officer’s technical representative and provided input during the implementation of the horizon scanning system. PCORI does not directly participate in horizon scanning or assessing leads or topics and did not provide opinions regarding potential impact of interventions. Financial Disclosure Statement None of the individuals compiling this information have any affiliations or financial involvement that conflicts with the material presented in this report. Public Domain Notice This document is in the public domain and may be used and reprinted without special permission. Citation of the source is appreciated. All statements, findings, and conclusions in this publication are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI) or its Board of Governors. -

Harnessing the Immune System to Prevent Cancer: Basic Immunologic Mechanisms & Their Application to Clinical Trials of Vaccines Part 2: the Vaccines Barbara K
Harnessing the Immune System to Prevent Cancer: Basic Immunologic Mechanisms & Their Application to Clinical Trials of Vaccines Part 2: The Vaccines Barbara K. Dunn NCI/Division of Cancer Prevention August 3, 2020 Harnessing the Immune System to Prevent Cancer: Basic Immunologic Mechanisms and Therapeutic Approaches that are Relevant to Cancer Prevention I. Basic immunologic mechanisms II. Application to prevention & treatment of cancer 1. Antibodies: as drugs 2. Vaccines: general principles & your vaccine trials & more… I) Vaccines to prevent cancers caused by infectious agents II) Vaccines to prevent non-infection associated cancer (directed toward tumor associated antigens) Harnessing the Immune System to Prevent Cancer: Basic Immunologic Mechanisms and Therapeutic Approaches that are Relevant to Cancer Prevention I. Basic immunologic mechanisms II. Application to prevention & treatment of cancer 1. Antibodies: as drugs “passive immunity” 2. Vaccines: general principles & your vaccine trials & more… I) Vaccines to prevent cancers caused by infectious agents “active II) Vaccines to prevent immunity” non-infection associated cancer (directed toward tumor associated antigens) Harnessing the Immune System to Prevent Cancer: Basic Immunologic Mechanisms and Therapeutic Approaches that are Relevant to Cancer Prevention I. Basic immunologic mechanisms II. Application to prevention & treatment of cancer 1. Antibodies:Focus as on drugs the Antigen “passive !immunity” 2. Vaccines: general principles & your vaccine trials & more… I) Vaccines -

The Tangible Effects of COVID-19 in Latin American Countries Boletín Del Colegio Mexicano De UROLOGIA´
Care and management in Urology oncology: The tangible effects of COVID-19 in Latin American countries Boletín del Colegio Mexicano de UROLOGIA´ BOLETÍN DEL COLEGIO MEXICANO DE UROLOGÍA, Vol. 36, año 2021, es una revista de publicación continua editada por el Colegio Mexicano de Urología Nacional, A.C., Montecito No. 38, Piso 33, Oficina 32, Col. Nápoles, C.P. 03810 CDMX, México. Tel. directo: (01-55) 9000-8053. http://www.cmu.org.mx Director: Dr. Héctor Berea Domínguez, Editor responsable: Dr. Erick Sierra Díaz, Co-Editores: Dr. Israel Presteguín Rosas, Dr. Rafael Sandoval Gómez, Asistente editorial: Lic. Angélica M. Arévalo Zacarías. Reservas de Derechos al Uso Exclusivo del título (04-2011-12081034400-106). ISNN: (0187-4829). Licitud de Título Núm. 016. Licitud de Contenido Núm. 008, de fecha 15 de agosto de 1979, ambos otorgados por la Comisión Clasifica- dora de Publicaciones y Revistas Ilustradas de la Secretaría de Gobernación. Los conceptos vertidos en los artículos publicados en este Bletín son responsabilidad exclusiva de los autores, y no reflejan necesariamente el criterio de el Colegio Mexicano de Urología Nacional, A.C. Este número se terminó de imprimir en abril de 2021. Publicación realizada, comercializada y distribuida por Edición y Farmacia SA de CV (Nieto Editores®). Cerrada de Antonio Maceo 68, colonia Escandón, 11800 Ciudad de México. Teléfono: 5678-2811. Queda estrictamente prohibida la reproducción total o parcial de los contenidos e imágenes de la publicación sin pevia auto- rización del Colegio Mexicano de Urología Nacional, A.C. Mesa Directiva 2019-2021 Dr. Ignacio López Caballero Presidente Dr. Héctor Raúl Vargas Zamora Vicepresidente Dr. -

Putting Into Perspective the Future of Cancer Vaccines: Targeted Immunotherapy
Putting into Perspective the Future of Cancer Vaccines: Targeted Immunotherapy Authors: Issam Makhoul,1,2 *Thomas Kieber-Emmons2,3 1. Department of Medicine, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA 2. Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA 3. Department of Pathology, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA *Correspondence to [email protected] Disclosure: The authors have declared no conflicts of interest. Received: 11.11.19 Accepted: 12.02.20 Keywords: Cancer, cancer vaccine, checkpoint inhibitors. Citation: EMJ. 2020;5[3]:102-113. Abstract Pre-clinical models and human clinical trials have confirmed the ability of cancer vaccines to induce immune responses that are tumour-specific and, in some cases, associated with clinical response. However, cancer vaccines as a targeted immunotherapy strategy have not yet come of age. So, why the discordance after so much research has been invested in cancer vaccines? There are several reasons for this that include: limited tumour immunogenicity (limited targeted antigen expression, antigen tolerance); antigenic heterogeneity in tumours; heterogeneity of individual immune responses; multiple mechanisms associated with suppressed functional activity of immune effector cells, the underlying rationale for the use of immune checkpoint inhibitors; and immune system exhaustion. The success of checkpoint therapy has refocussed investigations into defining relationships between tumours and host immune systems, appreciating the mechanisms by which tumour cells escape immune surveillance and reinforcing recognition of the potential of vaccines in the treatment and prevention of cancer. Recent developments in cancer immunotherapies, together with associated technologies, for instance, the unparalleled achievements by immune checkpoint inhibitors and neo- antigen identification tools, may foster potential improvements in cancer vaccines for the treatment of malignancies. -

ISSN: 2320-5407 Int. J. Adv. Res. 6(3), 1363-1370
ISSN: 2320-5407 Int. J. Adv. Res. 6(3), 1363-1370 Journal Homepage: -www.journalijar.com Article DOI:10.21474/IJAR01/6806 DOI URL: http://dx.doi.org/10.21474/IJAR01/6806 RESEARCH ARTICLE A CRITIQUE ON CANCER VACCINE. Sambathkumar R1, Amala Baby2, Sudha M3 and Venkateswaramurthy N2. 1. Department of Pharmaceutics. 2. Department of Pharmacy Practice. 3. Department of Pharmacology J.K.K. Nattraja College of Pharmacy, Kumarapalayam, Tamilnadu - 638183, India. …………………………………………………………………………………………………….... Manuscript Info Abstract ……………………. ……………………………………………………………… Manuscript History The goal of a successful vaccine is to prepare the immune system for invasion of a foreign pathogen and teach them to recognize antigens as Received: 21 January 2018 well as reduce the risk of transmission. In the field of cancer, vaccines Final Accepted: 23 February 2018 are found to be the latest discovery. Provenge® (Sipuleucel-T) is the Published: March 2018 only vaccine approved by Food and Drug Administration for the Keywords:- treatment of cancer. Vaccines are an appealing therapeutic strategy Cancer vaccine, Cells, Treatment. because they are specific. In addition they stimulate the adaptive immune system, thereby producing a memory response allowing for sustained effect without repeated therapy. Revelation of a potential anticancer treatment is as yet a test to the researchers. Thus, the development of effective cancer vaccines require, thoughtful clinical trials, and scientific progress which might induce long-term specific anticancer response and could contribute to effective and lasting elimination of malignant cells. Copy Right, IJAR, 2018,. All rights reserved. …………………………………………………………………………………………………….... Introduction:- Worldwide 6.7 million deaths were reported due to cancer in 2000. But the WHO has predicted this figure to be 15 million by 2020.[1, 2] Despite great efforts to develop better therapy, in 1997 more than 6 million people worldwide died from cancer. -

Immunological Approaches for Treatment of Advanced Stage Cancers Invariably Refractory to Drugs Talwar GP*, Jagdish C
C al & ellu ic la n r li Im C m f u Journal of o n l o a l n o r g Talwar et al., J Clin Cell Immunol 2014, 5:4 u y o J DOI: 10.4172/2155-9899.1000247 ISSN: 2155-9899 Clinical & Cellular Immunology Review Article Open Access Immunological Approaches for Treatment of Advanced Stage Cancers Invariably Refractory to Drugs Talwar GP*, Jagdish C. Gupta, Yogesh Kumar, Kripa N. Nand, Neha Ahlawat, Himani Garg, Kannagi Rana and Hilal Bhat Talwar Research Foundation, New Delhi, India *Corresponding author: Prof. G.P. Talwar, Talwar Research Foundation, E-8, Neb Valley Neb Sarai, New Delhi 110068, India, Tel: 91-011-65022405, 65022404; E- mail: [email protected] Received date: June 18, 2014, Accepted date: August 08, 2014, Published date: August 18, 2014 Copyright: © 2014 Talwar GP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Worldwide, deaths due to cancers are taking an increasing toll. Invariably over time cancer cells become refractory to available drugs. At this stage, the tumor is largely metastasized and not amenable to radical surgery or focal radiations. This review seeks to bring out the existence of heterogeneity of cell types in each cancer, and proposes adoption of a combined approach employing more than one therapeutic agent for a more lasting treatment. Also proposed is the use of monoclonal therapeutic antibodies and vaccines against ectopically expressed key molecules for killing of cancer cells and prevention of their multiplication. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

The Clinical Trial Landscape in Amyotrophic Lateral Sclerosis—Past, Present, and Future
Received: 16 September 2019 | Revised: 8 December 2019 | Accepted: 27 January 2020 DOI: 10.1002/med.21661 REVIEW ARTICLE The clinical trial landscape in amyotrophic lateral sclerosis—Past, present, and future Heike J. Wobst1 | Korrie L. Mack2,3 | Dean G. Brown4 | Nicholas J. Brandon1 | James Shorter2 1Neuroscience, BioPharmaceuticals R&D, AstraZeneca, Boston, Massachusetts Abstract 2 Department of Biochemistry and Biophysics, Amyotrophic lateral sclerosis (ALS) is a fatal neurodegen- Perelman School of Medicine, University of erative disease marked by progressive loss of muscle func- Pennsylvania, Philadelphia, Pennsylvania ‐ 3Merck & Co, Inc, Kenilworth, New Jersey tion. It is the most common adult onset form of motor 4Hit Discovery, Discovery Sciences, neuron disease, affecting about 16 000 people in the United BioPharmaceuticals R&D, AstraZeneca, Boston, States alone. The average survival is about 3 years. Only two Massachusetts interventional drugs, the antiglutamatergic small‐molecule Correspondence riluzole and the more recent antioxidant edaravone, have Heike J. Wobst, Jnana Therapeutics, Northern been approved for the treatment of ALS to date. Therapeutic Avenue, Boston, MA 02210. Email: [email protected] strategies under investigation in clinical trials cover a range of different modalities and targets, and more than 70 dif- James Shorter, Department of Biochemistry and Biophysics, Perelman School of Medicine, ferent drugs have been tested in the clinic to date. Here, we University of Pennsylvania, Philadelphia, PA summarize and classify interventional therapeutic strategies 19104. Email: [email protected] based on their molecular targets and phenotypic effects. We also discuss possible reasons for the failure of clinical trials in Present address Heike J. Wobst, Dean G. -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known.