Hair Loss in Children
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Fungal Infections from Human and Animal Contact
Journal of Patient-Centered Research and Reviews Volume 4 Issue 2 Article 4 4-25-2017 Fungal Infections From Human and Animal Contact Dennis J. Baumgardner Follow this and additional works at: https://aurora.org/jpcrr Part of the Bacterial Infections and Mycoses Commons, Infectious Disease Commons, and the Skin and Connective Tissue Diseases Commons Recommended Citation Baumgardner DJ. Fungal infections from human and animal contact. J Patient Cent Res Rev. 2017;4:78-89. doi: 10.17294/2330-0698.1418 Published quarterly by Midwest-based health system Advocate Aurora Health and indexed in PubMed Central, the Journal of Patient-Centered Research and Reviews (JPCRR) is an open access, peer-reviewed medical journal focused on disseminating scholarly works devoted to improving patient-centered care practices, health outcomes, and the patient experience. REVIEW Fungal Infections From Human and Animal Contact Dennis J. Baumgardner, MD Aurora University of Wisconsin Medical Group, Aurora Health Care, Milwaukee, WI; Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, Madison, WI; Center for Urban Population Health, Milwaukee, WI Abstract Fungal infections in humans resulting from human or animal contact are relatively uncommon, but they include a significant proportion of dermatophyte infections. Some of the most commonly encountered diseases of the integument are dermatomycoses. Human or animal contact may be the source of all types of tinea infections, occasional candidal infections, and some other types of superficial or deep fungal infections. This narrative review focuses on the epidemiology, clinical features, diagnosis and treatment of anthropophilic dermatophyte infections primarily found in North America. -

Pediatric and Adolescent Dermatology
Pediatric and adolescent dermatology Management and referral guidelines ICD-10 guide • Acne: L70.0 acne vulgaris; L70.1 acne conglobata; • Molluscum contagiosum: B08.1 L70.4 infantile acne; L70.5 acne excoriae; L70.8 • Nevi (moles): Start with D22 and rest depends other acne; or L70.9 acne unspecified on site • Alopecia areata: L63 alopecia; L63.0 alopecia • Onychomycosis (nail fungus): B35.1 (capitis) totalis; L63.1 alopecia universalis; L63.8 other alopecia areata; or L63.9 alopecia areata • Psoriasis: L40.0 plaque; L40.1 generalized unspecified pustular psoriasis; L40.3 palmoplantar pustulosis; L40.4 guttate; L40.54 psoriatic juvenile • Atopic dermatitis (eczema): L20.82 flexural; arthropathy; L40.8 other psoriasis; or L40.9 L20.83 infantile; L20.89 other atopic dermatitis; or psoriasis unspecified L20.9 atopic dermatitis unspecified • Scabies: B86 • Hemangioma of infancy: D18 hemangioma and lymphangioma any site; D18.0 hemangioma; • Seborrheic dermatitis: L21.0 capitis; L21.1 infantile; D18.00 hemangioma unspecified site; D18.01 L21.8 other seborrheic dermatitis; or L21.9 hemangioma of skin and subcutaneous tissue; seborrheic dermatitis unspecified D18.02 hemangioma of intracranial structures; • Tinea capitis: B35.0 D18.03 hemangioma of intraabdominal structures; or D18.09 hemangioma of other sites • Tinea versicolor: B36.0 • Hyperhidrosis: R61 generalized hyperhidrosis; • Vitiligo: L80 L74.5 focal hyperhidrosis; L74.51 primary focal • Warts: B07.0 verruca plantaris; B07.8 verruca hyperhidrosis, rest depends on site; L74.52 vulgaris (common warts); B07.9 viral wart secondary focal hyperhidrosis unspecified; or A63.0 anogenital warts • Keratosis pilaris: L85.8 other specified epidermal thickening 1 Acne Treatment basics • Tretinoin 0.025% or 0.05% cream • Education: Medications often take weeks to work AND and the patient’s skin may get “worse” (dry and red) • Clindamycin-benzoyl peroxide 1%-5% gel in the before it gets better. -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -
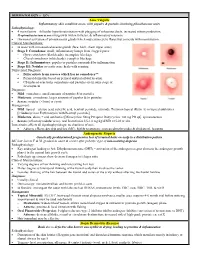
Pediatrics-EOR-Outline.Pdf
DERMATOLOGY – 15% Acne Vulgaris Inflammatory skin condition assoc. with papules & pustules involving pilosebaceous units Pathophysiology: • 4 main factors – follicular hyperkeratinization with plugging of sebaceous ducts, increased sebum production, Propionibacterium acnes overgrowth within follicles, & inflammatory response • Hormonal activation of pilosebaceous glands which may cause cyclic flares that coincide with menstruation Clinical Manifestations: • In areas with increased sebaceous glands (face, back, chest, upper arms) • Stage I: Comedones: small, inflammatory bumps from clogged pores - Open comedones (blackheads): incomplete blockage - Closed comedones (whiteheads): complete blockage • Stage II: Inflammatory: papules or pustules surrounded by inflammation • Stage III: Nodular or cystic acne: heals with scarring Differential Diagnosis: • Differentiate from rosacea which has no comedones** • Perioral dermatitis based on perioral and periorbital location • CS-induced acne lacks comedones and pustules are in same stage of development Diagnosis: • Mild: comedones, small amounts of papules &/or pustules • Moderate: comedones, larger amounts of papules &/or pustules • Severe: nodular (>5mm) or cystic Management: • Mild: topical – azelaic acid, salicylic acid, benzoyl peroxide, retinoids, Tretinoin topical (Retin A) or topical antibiotics [Clindamycin or Erythromycin with Benzoyl peroxide] • Moderate: above + oral antibiotics [Minocycline 50mg PO qd or Doxycycline 100 mg PO qd], spironolactone • Severe (refractory nodular acne): oral -

Rozex Cream Ds
New Zealand Datasheet 1 PRODUCT NAME ROZEX® CREAM 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Metronidazole 7.5 mg/g 3 PHARMACEUTICAL FORM Contains 0.75% w/w metronidazole as the active ingredient in an oil-in-water cream base containing 4 CLINICAL PARTICULARS 4.1 Therapeutic indications ROZEX CREAM is indicated for the treatment inflammatory papules and pustules of rosacea. 4.2 Dosage and method of administration ROZEX CREAM should be applied in a thin layer to the affected areas of the skin twice daily, morning and evening. Areas to be treated should be washed with a mild cleanser before application. Patients may use non-comedogenic and non-astringent cosmetics after application of ROZEX CREAM. The dosage does not need to be adjusted for elderly patients. Safety and effectiveness in paediatric patients have not been established. ROZEX is not recommended for use in children. The average period of treatment is three to four months. The recommended duration of treatment should not be exceeded. If a clear benefit has been demonstrated continued therapy for a further three to four months period may be considered by the prescribing physician depending upon the severity of the condition. Clinical experience with ROZEX CREAM over prolonged periods is limited at present. Patients should be monitored to ensure that clinical benefit continues and that no local or systemic events occur. In the absence of a clear clinical improvement therapy should be stopped. ROZEX CREAM should not be used in or close to the eyes. The use of a sunscreen is recommended when exposure to sunlight cannot be avoided. -

Herb Lotions to Regrow Hair in Patients with Intractable Alopecia Areata Hideo Nakayama*, Ko-Ron Chen Meguro Chen Dermatology Clinic, Tokyo, Japan
Clinical and Medical Investigations Research Article ISSN: 2398-5763 Herb lotions to regrow hair in patients with intractable alopecia areata Hideo Nakayama*, Ko-Ron Chen Meguro Chen Dermatology Clinic, Tokyo, Japan Abstract The history of herbal medicine in China goes back more than 1,000 years. Many kinds of mixtures of herbs that are effective to diseases or symptoms have been transmitted from the middle ages to today under names such as Traditional Chinese Medicine (TCM) in China and Kampo in Japan. For the treatment of severe and intractable alopecia areata, such as alopecia universalis, totalis, diffusa etc., herb lotions are known to be effective in hair regrowth. Laiso®, Fukisin® in Japan and 101® in China are such effective examples. As to treat such cases, systemic usage of corticosteroid hormones are surely effective, however, considering their side effects, long term usage should be refrained. There are also these who should refrain such as small children, and patients with peptic ulcers, chronic infections and osteoporosis. AL-8 and AL-4 were the prescriptions removing herbs which are not allowed in Japanese Pharmacological regulations from 101, and salvia miltiorrhiza radix (SMR) is the most effective herb for hair growth, also the causation to produce contact sensitization. Therefore, the mechanism of hair growth of these herb lotions in which the rate of effectiveness was in average 64.8% on 54 severe intractable cases of alopecia areata, was very similar to DNCB and SADBE. The most recommended way of developing herb lotion with high ability of hairgrowth is to use SMR but its concentration should not exceed 2%, and when sensitization occurs, the lotion should be changed to Laiso® or Fukisin®, which do not contain SMR. -

Concomitant Manifestation of Pili Annulati and Alopecia Areata: Coincidental Rather Than True Association
Acta Derm Venereol 2011; 91: 459–462 CLINICAL REPORT Concomitant Manifestation of Pili Annulati and Alopecia Areata: Coincidental Rather than True Association Kathrin A. GIEHL1, Matthias SCHMUTH2, Antonella TOSTI3, David A. De BERKER4, Alexander CRISPIN5, Hans WOLFF1 and Jorge FRANK6,7 Department of Dermatology, 1Ludwig-Maximilian University, Munich, Germany, 2Medical University Innsbruck, Austria, 3University of Bologna, Italy, 4University of Bristol, UK, 5Department of Medical Informatics, Biometry and Epidemiology, Ludwig-Maximilian University, Munich, Germany, 6Depart- ment of Dermatology, and 7GROW – School for Oncology and Developmental Biology, Maastricht University Medical Center (MUMC), The Netherlands The autosomal dominantly inherited hair disorder pili with these cavities, most patients with PA do not report annulati is characterized by alternating light and dark increased hair fragility (5, 6). bands of the hair shaft. Concomitant manifestation of A locus for PA has been shown on chromosome pili annulati with alopecia areata has been reported 12p24.32–24.33 (7, 8). A previous study of hair follicle previously on several occasions. However, no systematic morphology indicates that the disease may arise from evaluation of patients manifesting both diseases has been a defect that putatively affects a regulatory element performed. We studied the simultaneous or sequential involved in the assembly of structural proteins within occurrence of pili annulati and alopecia areata in indi- the extracellular matrix (9). viduals diagnosed in different European academic der- In contrast to PA, alopecia areata (AA) is a polyge- matology units. We included 162 Caucasian individuals netic, immune-mediated disorder of the hair follicle from 14 extended families, comprising 76 affected and that has an unpredictable, and sometimes self-limiting, 86 unaffected family members. -

Short Anagen Syndrome: a Case Study
Journal of Cosmetics, Dermatological Sciences and Applications, 2012, 2, 14-15 http://dx.doi.org/10.4236/jcdsa.2012.21004 Published Online March 2012 (http://www.SciRP.org/journal/jcdsa) Short Anagen Syndrome: A Case Study Martina Alés Fernández, Francisco M. Camacho Martínez Department of Dermatology, Virgen Macarena University Hospital, Seville, Spain. Email: [email protected], [email protected] Received October 31st, 2011; revised November 18th, 2011; revised November 29th, 2011 ABSTRACT Short anagen syndrome is a relatively recently described entity. This syndrome is an unusual condition where the ana- gen growth phase of hair follicles is shorter than normal. Its clinical characteristics and trichogram findings contribute to the diagnosis of this trichosis. Keywords: Anagen Syndrome 1. Case Report Three-years-old girl with low density and slow growth scalp hair that had not been cut since birth. Her birth and medical history were unremarkable. The physical ex- amination revealed short and fine brown scalp hair with decreased density in frontoparietal areas (Figure 1). The rest of the physical examination was normal, without any abnormalities in eyelashes, eyebrows, teeth, nails or skin. The hair pull test was negative. The trichogram demon- strated some dystrophic hairs, but the most important data was an increased number of telogen hairs with a consistent decreased number of anagen hairs (Figure 2). The anagen to telogen ratio (7:28) was significantly re- duced with only 25% of hairs in anagen. 2. Discussion Short anagen syndrome is a relatively recently recog- nized entity poorly documented. Short hair due to a short anagen phase was described in 1987 by Kersey as part of tricho-dental syndrome [1]. -

Alopecia, Particularly: Alopecia Areata Androgenetic Alopecia Telogen Effluvium Anagen Effluvium
432 Teams Dermatology Hair disorders Color Code: Original, Team’s note, Important, Doctor’s note, Not important, Old teamwork Done by: Shaikha Aldossari Reviewer: Lama AlTawil 8 Team Leader: Basil Al Suwaine&Lama Al Tawil 432 Dermatology Team Lecture 8: Hair Disorders Objectives 1- Normal anatomy of hair follicle and hair cycle. 2- Causes, features and management of non scarring alopecia, particularly: Alopecia areata Androgenetic alopecia Telogen effluvium Anagen effluvium 3- Causes and features of scarring alopecia. 4- Causes and features of Excessive hair growth. hair disorder Excessive hair Alopecia growth non scarring Hirsutism Hypertrichosis scarring Anagen Telogen Androgenetic Alopecia effluvium effluvium Alopecia Areata P a g e | 1 432 Dermatology Team Lecture 8: Hair Disorders Anatomy of hair follicle: The Arrector piliResponsible for piloerection (goose bumps ) that happens when one is cold (produces energy and therefor warmth) . hair follicle becomes vertical instead of oblique Cuticle is the last layer here . what we can see outside . it has 7 layers of keratinocytes How many hairs in the body? 5 millions hairs in the body, 100,000 in the scalp. Growth rate: 0.3mm/day for scalp hair i.e.1cm/month Hair follicle bulge: -Very important part since it has stem cells .its the inertion of the arrector pili Hair follicle on vertical section: -So any pathological process affecting any part other Initially the shaft and the follicle are one than this, hair would still be able to regrow. organ then when you reach 1/3 the follicle -If we want to destroy a hair follicle, we’d target the bulge. -

Acquired Hypertrichosis of the Periorbital Area and Malar Cheek
PHOTO CHALLENGE Acquired Hypertrichosis of the Periorbital Area and Malar Cheek Caitlin G. Purvis, BS; Justin P. Bandino, MD; Dirk M. Elston, MD An otherwise healthy woman in her late 50s with Fitzpatrick skin type II presented to the derma- tology department for a scheduled cosmetic botulinum toxin injection. Her medical history was notable only for periodic nonsurgical cosmetic procedures including botulinum toxin and dermal fillers, and she was not taking any daily systemic medications. Duringcopy the preoperative assess- ment, subtle bilateral and symmetric hypertricho- sis with darker terminal hair formation was noted on the periorbital skin and zygomatic cheek. Uponnot inquiry, the patient admitted to purchas- ing a “special eye drop” from Mexico and using it regularly. After instillation of 2 to 3 drops per eye, she would laterally wipe the resulting excess Dodrops away from the eyes with her hands and then wash her hands. She denied a change in eye color from their natural brown but did report using blue color contact lenses. She denied an increase in hair growth elsewhere including the upper lip, chin, upper chest, forearms, and hands. She denied deepening of her voice, CUTIS acne, or hair thinning. WHAT’S THE DIAGNOSIS? a. acetazolamide-induced hypertrichosis b. betamethasone-induced hypertrichosis c. bimatoprost-induced hypertrichosis d. cyclosporine-induced hypertrichosis e. timolol-induced hypertrichosis PLEASE TURN TO PAGE E21 FOR THE DIAGNOSIS From the Department of Dermatology, Medical University of South Carolina, Charleston. The authors report no conflict of interest. Correspondence: Justin P. Bandino, MD, 171 Ashley Ave, MSC 908, Charleston, SC 29425 ([email protected]). -

Hypertrichosis in Alopecia Universalis and Complex Regional Pain Syndrome
NEUROIMAGES Hypertrichosis in alopecia universalis and complex regional pain syndrome Figure 1 Alopecia universalis in a 46-year- Figure 2 Hypertrichosis of the fifth digit of the old woman with complex regional complex regional pain syndrome– pain syndrome I affected hand This 46-year-old woman developed complex regional pain syndrome (CRPS) I in the right hand after distor- tion of the wrist. Ten years before, the diagnosis of alopecia areata was made with subsequent complete loss of scalp and body hair (alopecia universalis; figure 1). Apart from sensory, motor, and autonomic changes, most strikingly, hypertrichosis of the fifth digit was detectable on the right hand (figure 2). Hypertrichosis is common in CRPS.1 The underlying mechanisms are poorly understood and may involve increased neurogenic inflammation.2 This case nicely illustrates the powerful hair growth stimulus in CRPS. Florian T. Nickel, MD, Christian Maiho¨fner, MD, PhD, Erlangen, Germany Disclosure: The authors report no disclosures. Address correspondence and reprint requests to Dr. Florian T. Nickel, Department of Neurology, University of Erlangen-Nuremberg, Schwabachanlage 6, 91054 Erlangen, Germany; [email protected] 1. Birklein F, Riedl B, Sieweke N, Weber M, Neundorfer B. Neurological findings in complex regional pain syndromes: analysis of 145 cases. Acta Neurol Scand 2000;101:262–269. 2. Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology 2001;57:2179–2184. Copyright © 2010 by -

Revisiting Hair Follicle Embryology, Anatomy and the Follicular Cycle
etology sm & o C T r f i o c h l o a l n o r Silva et al., J Cosmo Trichol 2019, 5:1 g u y o J Journal of Cosmetology & Trichology DOI: 10.4172/2471-9323.1000141 ISSN: 2471-9323 Review Article Open Access Revisiting Hair Follicle Embryology, Anatomy and the Follicular Cycle Laura Maria Andrade Silva1*, Ricardo Hsieh2, Silvia Vanessa Lourenço3, Bruno de Oliveira Rocha1, Ricardo Romiti4, Neusa Yuriko Sakai Valente4,5, Alessandra Anzai4 and Juliana Dumet Fernandes1 1Department of Dermatology, Magalhães Neto Outpatient Clinic, Professor Edgar Santos School Hospital Complex, The Federal University of Bahia (C-HUPES/UFBa), Salvador/BA-Brazil 2Institute of Tropical Medicine of São Paulo, The University of São Paulo (IMT/USP), São Paulo/SP-Brazil 3Department of Stomatology, School of Dentistry, The University of São Paulo (FO/USP), São Paulo/SP-Brazil 4Department of Dermatology, University of São Paulo Medical School, The University of São Paulo (FMUSP), São Paulo/SP-Brazil 5Department of Dermatology, Hospital of the Public Server of the State of São Paulo (IAMSPE), São Paulo/SP-Brazil *Corresponding author: Laura Maria Andrade Silva, Department of Dermatology, Magalhães Neto Outpatient Clinic, Professor Edgar Santos School Hospital Complex, The Federal University of Bahia (C-HUPES/UFBa) Salvador/BA-Brazil, Tel: +55(71)3283-8380; +55(71)99981-7759; E-mail: [email protected] Received date: January 17, 2019; Accepted date: March 07, 2019; Published date: March 12, 2019 Copyright: © 2019 Silva LMA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.