Nanoscape, a Data-Driven 3D Real-Time Interactive Virtual Cell Environment
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Debian GNU/Linux 4.0 (“Etch”) (Mips )
Debian GNU/Linux 4.0 (“etch”) JJー スノー( (Mips 用) Josip Rodin, Bob Hilliard, Adam Di Carlo, Anne Bezemer, Rob Bradford, Frans Pop (現在.AS&*), Andreas Barth (現在.AS&*), Javier Fernández-Sanguino Peña (現在.AS&*), Steve Langasek (現在.AS&*) <[email protected]> $Id: release-notes.en.sgml,v 1.312 2007-08-16 22:24:38 jseidel Exp $ i 目目目 hhh 1 //はじじじめAA+++ 1 1.1 この£書+¯するバグR報告する ...........................1 1.2 アップグレー)についての報告をする .........................2 1.3 この£書.ソース .....................................2 2 Debian GNU/Linux 4.0 ...最最最新新新情ss報報報 3 2.1 Mips +¯する最新情報 .................................4 2.2 ディストリビューション.最新情報 ..........................4 2.2.1 パッケージ管理 ..................................5 2.2.2 debian-volatile がGwサービス+ .......................6 2.3 システム.改, ......................................6 2.4 カー-K¯c.®要*¼f点 ..............................7 2.4.1 カーネルパッケージングにおける¼f ....................8 2.4.2 新しい initrd 生成Fーティリティ .......................8 2.4.3 #¿* /dev 管理(/ードウェア検õ .....................8 3 イイインSンスススト((ーーーKKルシシシススステ&&@@@ 11 3.1 インストールシステム.最新情報 ........................... 11 3.1.1 ®要*¼f点 ................................... 11 3.1.2 r#インストーK ................................ 13 3.2 ®気コンテスト ...................................... 14 4 )))MMM...JJJJJJーーースススかかからIIアアアッ##77プグググレLLーーー)))すすするKK 15 4.1 アップグレー).準& .................................. 15 4.1.1 あらゆる'ータD設¡s報Rバックアップする ............... 15 目 h ii 4.1.2 _M+Fーザ+通知する ............................ 16 4.1.3 復!.準& .................................... 16 4.1.4 アップグレー)用.!Q*環@.準& .................... 17 4.1.5 2.2 系カー-K/サポー(されなくなりました ................ 17 4.2 システム.x態Rチェックする ............................. 17 4.2.1 パッケージマネージャU.? ?.アクションRGx ........... 17 4.2.2 APT . pin 機能R\Iにする .......................... 18 4.2.3 パッケージ.x態Rチェックする ....................... 18 4.2.4 "Gw*ソース(バックポー( ........................ 19 4.3 パッケージ.>ークR±ù[')す ......................... -

Windows Subsystem for Linux
LINUX 101 ... FOR .NET DEVS Oliver Sturm • @olivers • [email protected] OLIVER STURM Training Director at DevExpress Consultant, trainer, author, software architect and developer for over 25 years Contact: [email protected] Linux 101 2 / 37 AGENDA That Linux Thing Getting Started with Linux Shells, Command Lines and Commands File Systems and Permissions Users and Processes Editing and Configuring Packages Creating a .NET Core App Setting Up a Runtime Environment Linux 101 3 / 37 ON DAY 1... From: Linus Benedict Torvalds Date: August 25 1991 Subject: What would you like to see most in minix? Hello everybody out there using minix - I'm doing a (free) operating system (just a hobby, won't be big and professional like gnu) for 386(486) AT clones. PS. It is NOT protable (uses 386 task switching etc), and it probably never will support anything other than AT-harddisks, as that's all I have :-(. Full thread: http://osturm.me/torvalds-linux-announcement Linux 101 4 / 37 ON DAY 1... From: Linus Benedict Torvalds Date: August 25 1991 Subject: What would you like to see most in minix? Hello everybody out there using minix - Y THE AY I'm Bdoin g a ( Wfree) operating system (just a hobby, won't be big and professional like gnu) for 386(486) AT clones. Linus doesn't mention it, but his new OS was going to be PS. called It Freaxis NO Tat p thisrota bpoint.le (uses 386 task switching etc), and it probably never will support anything other than AT-harddisks, as that's all I have :-(. -

Praise for the Official Ubuntu Book
Praise for The Official Ubuntu Book “The Official Ubuntu Book is a great way to get you started with Ubuntu, giving you enough information to be productive without overloading you.” —John Stevenson, DZone Book Reviewer “OUB is one of the best books I’ve seen for beginners.” —Bill Blinn, TechByter Worldwide “This book is the perfect companion for users new to Linux and Ubuntu. It covers the basics in a concise and well-organized manner. General use is covered separately from troubleshooting and error-handling, making the book well-suited both for the beginner as well as the user that needs extended help.” —Thomas Petrucha, Austria Ubuntu User Group “I have recommended this book to several users who I instruct regularly on the use of Ubuntu. All of them have been satisfied with their purchase and have even been able to use it to help them in their journey along the way.” —Chris Crisafulli, Ubuntu LoCo Council, Florida Local Community Team “This text demystifies a very powerful Linux operating system . in just a few weeks of having it, I’ve used it as a quick reference a half dozen times, which saved me the time I would have spent scouring the Ubuntu forums online.” —Darren Frey, Member, Houston Local User Group This page intentionally left blank The Official Ubuntu Book Sixth Edition This page intentionally left blank The Official Ubuntu Book Sixth Edition Benjamin Mako Hill Matthew Helmke Amber Graner Corey Burger With Jonathan Jesse, Kyle Rankin, and Jono Bacon Upper Saddle River, NJ • Boston • Indianapolis • San Francisco New York • Toronto • Montreal • London • Munich • Paris • Madrid Capetown • Sydney • Tokyo • Singapore • Mexico City Many of the designations used by manufacturers and sellers to distinguish their products are claimed as trademarks. -

Ubuntu Server Guide Basic Installation Preparing to Install
Ubuntu Server Guide Welcome to the Ubuntu Server Guide! This site includes information on using Ubuntu Server for the latest LTS release, Ubuntu 20.04 LTS (Focal Fossa). For an offline version as well as versions for previous releases see below. Improving the Documentation If you find any errors or have suggestions for improvements to pages, please use the link at thebottomof each topic titled: “Help improve this document in the forum.” This link will take you to the Server Discourse forum for the specific page you are viewing. There you can share your comments or let us know aboutbugs with any page. PDFs and Previous Releases Below are links to the previous Ubuntu Server release server guides as well as an offline copy of the current version of this site: Ubuntu 20.04 LTS (Focal Fossa): PDF Ubuntu 18.04 LTS (Bionic Beaver): Web and PDF Ubuntu 16.04 LTS (Xenial Xerus): Web and PDF Support There are a couple of different ways that the Ubuntu Server edition is supported: commercial support and community support. The main commercial support (and development funding) is available from Canonical, Ltd. They supply reasonably- priced support contracts on a per desktop or per-server basis. For more information see the Ubuntu Advantage page. Community support is also provided by dedicated individuals and companies that wish to make Ubuntu the best distribution possible. Support is provided through multiple mailing lists, IRC channels, forums, blogs, wikis, etc. The large amount of information available can be overwhelming, but a good search engine query can usually provide an answer to your questions. -

07 07 Unixintropart2 Lucio Week 3
Unix Basics Command line tools Daniel Lucio Overview • Where to use it? • Command syntax • What are commands? • Where to get help? • Standard streams(stdin, stdout, stderr) • Pipelines (Power of combining commands) • Redirection • More Information Introduction to Unix Where to use it? • Login to a Unix system like ’kraken’ or any other NICS/ UT/XSEDE resource. • Download and boot from a Linux LiveCD either from a CD/DVD or USB drive. • http://www.puppylinux.com/ • http://www.knopper.net/knoppix/index-en.html • http://www.ubuntu.com/ Introduction to Unix Where to use it? • Install Cygwin: a collection of tools which provide a Linux look and feel environment for Windows. • http://cygwin.com/index.html • https://newton.utk.edu/bin/view/Main/Workshop0InstallingCygwin • Online terminal emulator • http://bellard.org/jslinux/ • http://cb.vu/ • http://simpleshell.com/ Introduction to Unix Command syntax $ command [<options>] [<file> | <argument> ...] Example: cp [-R [-H | -L | -P]] [-fi | -n] [-apvX] source_file target_file Introduction to Unix What are commands? • An executable program (date) • A command built into the shell itself (cd) • A shell program/function • An alias Introduction to Unix Bash commands (Linux) alias! crontab! false! if! mknod! ram! strace! unshar! apropos! csplit! fdformat! ifconfig! more! rcp! su! until! apt-get! cut! fdisk! ifdown! mount! read! sudo! uptime! aptitude! date! fg! ifup! mtools! readarray! sum! useradd! aspell! dc! fgrep! import! mtr! readonly! suspend! userdel! awk! dd! file! install! mv! reboot! symlink! -

Working with Ubuntu Linux
Working with Ubuntu Linux Track 2 Workshop June 2010 Pago Pago, American Samoa Assumptions You are comfortable with the following: • Core Linux concepts - Shells - Permissions - Graphical user interface (GUI) vs. command line interface (CLI) • Working on the Linux command line • Editing files in Linux (using vi, nano or other text editors) • Basics of networking Is this correct? Goal Present the “Ubuntu philosophy” and differences from other Linux distributions. Including: • Naming conventions • Release conventions (Server, Desktop and LTS) • Other flavors • The Debian way • Packaging system (how software is installed) • Meta-packages • Keeping up-to-date • Stopping and starting services Ubuntu Timeline The Debian Way Ubuntu is built from Debian repositories and uses the Debian package management system. • Debian is a very cautious and strict Linux distribution: • Minimal new versions • Extremely well-tested • No closed source software • Beta version of Debian as stable as release quality for most Linux distributions. • New versions are not released until they are ready. • Latest versions of software often not available in main branch as they are not considered stable or safe enough. • There are pluses and minuses to this approach. The Ubuntu Take on the Debian Way Potentially heretical slide … • Use the Debian software repository concept to classify software. • Use the Debian package management system. • Be more open – Ubuntu allows closed source software and drivers. • Ubuntu pushes releases out fast, but supports releases for 2 to 5 years (Unlike Fedora Core’s 18 months). • Ubuntu aiming at both the desktop and server markets. • The “Ubuntu Project” is supported by Mark Shuttleworth. • Make maintaining a current system very easy to completely automatic (much like Windows). -

MX-19.2 Users Manual
MX-19.2 Users Manual v. 20200801 manual AT mxlinux DOT org Ctrl-F = Search this Manual Ctrl+Home = Return to top Table of Contents 1 Introduction...................................................................................................................................4 1.1 About MX Linux................................................................................................................4 1.2 About this Manual..............................................................................................................4 1.3 System requirements..........................................................................................................5 1.4 Support and EOL................................................................................................................6 1.5 Bugs, issues and requests...................................................................................................6 1.6 Migration............................................................................................................................7 1.7 Our positions......................................................................................................................8 1.8 Notes for Translators.............................................................................................................8 2 Installation...................................................................................................................................10 2.1 Introduction......................................................................................................................10 -

Debian Secrets Power Tools for Power Users
About this talk Changing system behaviour Searching Fooling the package system Anticipating and correcting problems Debian menu Debian Secrets Power tools for Power users Wouter Verhelst FOSDEM 2010 Wouter Verhelst Debian Secrets Idea: compare Debian-specic features against other distributions' features Help! About this talk Changing system behaviour Searching Fooling the package system Anticipating and correcting problems Debian menu About this talk Recycled from DebConf8 DebianDay Wouter Verhelst Debian Secrets Help! About this talk Changing system behaviour Searching Fooling the package system Anticipating and correcting problems Debian menu About this talk Recycled from DebConf8 DebianDay Idea: compare Debian-specic features against other distributions' features Wouter Verhelst Debian Secrets About this talk Changing system behaviour Searching Fooling the package system Anticipating and correcting problems Debian menu About this talk Recycled from DebConf8 DebianDay Idea: compare Debian-specic features against other distributions' features Help! Wouter Verhelst Debian Secrets update-alternatives −−config x-terminal-emulator more info: man update-alternatives About this talk Changing system behaviour Searching update-alternatives Fooling the package system dpkg-statoverride Anticipating and correcting problems Debian menu update-alternatives multiple packages providing a particular command Wouter Verhelst Debian Secrets more info: man update-alternatives About this talk Changing system behaviour Searching update-alternatives Fooling -
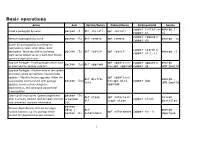
Basic Operations
Basic operations Action Arch Red Hat/Fedora Debian/Ubuntu SLES/openSUSE Gentoo zypper install or emerge [- Install a package(s) by name pacman -S dnf install apt install zypper in a] zypper remove or Remove a package(s) by name pacman -Rs dnf remove apt remove emerge -vc zypper rm Search for package(s) by searching the expression in name, description, short zypper search or description. What exact fields are being pacman -Ss dnf search apt search emerge -S zypper se [-s] searched by default varies in each tool. Mostly options bring tools on par. Upgrade Packages - Install packages which have apt update and zypper update or emerge - pacman -Syu dnf upgrade an older version already installed then apt upgrade zypper up uDN @world Upgrade Packages - Another form of the update command, which can perform more complex updates -- like distribution upgrades. When the apt update and dnf distro- emerge - usual update command will omit package pacman -Syu then apt dist- zypper dup sync uDN @world updates, which include changes in upgrade dependencies, this command can perform those updates. Clean up all local caches. Options might limit pacman -Sc dnf clean apt autoclean eclean what is actually cleaned. autoclean removes or pacman - zypper clean all or apt clean distfiles only unneeded, obsolete information. Scc pacman - Remove dependencies that are no longer Qdtq | dnf emerge -- needed, because e.g. the package which apt autoremove zypper rm -u pacman -Rs autoremove depclean needed the dependencies was removed. - 1 pacman -Qmq dnf Remove packages no longer included in any aptitude purge | pacman - repoquery -- repositories. -

Dpkg and APT First...What Is a Package? ● a Collection of Files That Is Installed on a Linux System
Package management: dpkg and APT First...what is a package? ● a collection of files that is installed on a Linux system ● usually distributed as compressed single files ○ tarballs ○ zip files ● the way software is distributed, installed, updated on a Linux system jboydt :: csci 26 | dpkg, apt And what is package management? ● a system for tracking all information about a software package ○ all of the files associated with the package ○ dependencies (such as specific versions of specific files) of the package ○ checksums to verify the validity of package files ○ a way to upgrade packages ○ a way to uninstall packages ● a system for obtaining software in its original source code form ○ then, building it and installing it on a specific machine jboydt :: csci 26 | dpkg, apt Package management on popular distros ● RedHat -- rpm/yum ○ also CentOS, SUSE ● Debian -- apt/aptitude ○ also Ubuntu ● ArchLinux -- pacman ● BSDs -- each its own ○ FreeBSD -- pkgng jboydt :: csci 26 | dpkg, apt dpkg jboydt :: csci 26 | dpkg, apt What is dpkg? ● the core of the Debian package management system ● requires .deb packages on local machine ● does not manage dependencies between packages jboydt :: csci 26 | dpkg, apt using dpkg ● common dpkg command options: ○ -i install ○ -r remove, leave config files ○ -P remove, remove config files ○ -p print information about an installed package ○ -l PATTERN list packages whose names match PATTERN * dpkg OPTION PACKAGE_NAME (add .deb to PACKAGE_NAME on install) jboydt :: csci 26 | dpkg, apt APT jboydt :: csci 26 | dpkg, apt What is APT? ● Advanced Packaging Tool ● advancements over dpkg ○ can fetch remote packages ■ remote targets configured in /etc/apt/sources.list ○ can manage package dependencies ○ can manage package upgrades ● a set of tools ○ apt-cache ○ apt-get ● one of the best things about Debian! jboydt :: csci 26 | dpkg, apt apt-cache ● apt-cache showpkg PACKAGE_NAME ○ display info about PACKAGE_NAME ● apt-cache stats ○ how many packages are installed, et al. -

Install Gnome Software Center Arch
Install gnome software center arch Upstream URL: License(s): GPL2. Maintainers: Jan Steffens. Package Size: MB. Installed Size: Installed Size: MB. gnome-software will be available as a preview in It can install, remove applications on systems with PackageKit. It can install updates on Gnome software will not start / Applications & Desktop. A quick video on Gnome Software Center in Arch Linux. Gnome unstable repository. There is a component called Polkit that is used by many applications to request root permissions to do things (it can do so because it's a. GNOME Software on #archlinux with native PackageKit backend, and this is a gui for installing software, ala ubuntu software manager, but distro This is some kind of Ubuntu Software Centre, with comments and all that. Need help installing Gnome Software Center for Arch Linux? Here are some instructions: Click DOWNLOAD HERE in the menu. Download the file. Make the file. I had to install it with along with packagekit. This is what's missing to make Antergos *the* beginner-friendly Arch-based distro, or general So, it is not a bad idea for the “Gnome Software Center” to include by default. GNOME software software center graphic that we will find the default in future releases of Fedora in addition to being installed in Arch Linux Please help me to install GNOME Software on. GNOME Software Will Work On Arch Linux With PackageKit the Alpm/Pacman back-end for using this GNOME application to install and. From: Sriram Ramkrishna ; To: desktop-devel-list devel-list gnome org>; Subject: gnome- software/packagekit. -

Pdf-Preview.Pdf
The Debian Administrator's Handbook Raphaël Hertzog and Roland Mas Copyright © 2003-2013 Raphaël Hertzog Copyright © 2006-2013 Roland Mas Copyright © 2012-2013 Freexian SARL ISBN: 979-10-91414-02-9 (English paperback) ISBN: 979-10-91414-03-6 (English ebook) This book is available under the terms of two licenses compatible with the Debian Free Software Guide- lines. Creative Commons License Notice: This book is licensed under a Creative Commons Attribution- ShareAlike 3.0 Unported License. è http://creativecommons.org/licenses/by-sa/3.0/ GNU General Public License Notice: This book is free documentation: you can redistribute it and/or modify it under the terms of the GNU General Public License as published by the Free Software Founda- tion, either version 2 of the License, or (at your option) any later version. This book is distributed in the hope that it will be useful, but WITHOUT ANY WARRANTY; without even the implied warranty of MERCHANTABILITY or FITNESS FOR A PARTICULAR PURPOSE. See the GNU Gen- eral Public License for more details. You should have received a copy of the GNU General Public License along with this program. If not, see http://www.gnu.org/licenses/. Show your appreciation This book is published under a free license because we want everybody to benefit from it. That said maintaining it takes time and lots of efforts, and we appreciate being thanked for this. If you find this book valuable, please consider contributing to its continued maintenance either by buying a pa- perback copy or by making a donation through the book's