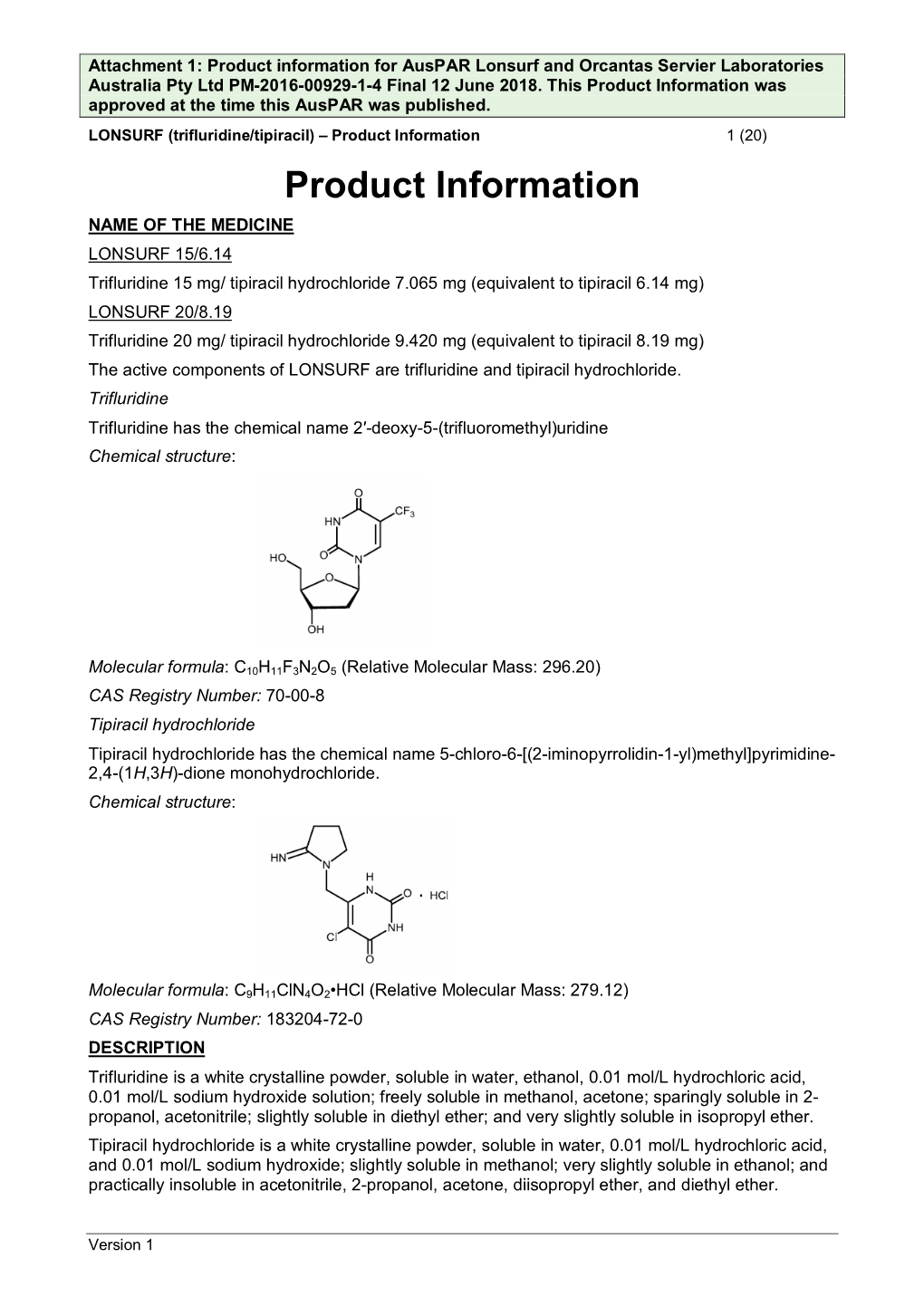
Auspar Attachment 1: Product Information: Trifluridine/Tipiracil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lonsurf (Trifluridine/Tipiracil) Were Bone Marrow Suppression and Gastrointestinal Toxicity
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION PrLONSURF® trifluridine and tipiracil tablet 15 mg trifluridine/ 6.14 mg tipiracil (as tipiracil hydrochloride) 20 mg trifluridine / 8.19 mg tipiracil (as tipiracil hydrochloride) Antineoplastic Agent Thymidine phosphorylase inhibitor/nucleoside metabolic inhibitor Taiho Pharma Canada, Inc. Date of Initial Approval: 2010 Winston Park Drive, Suite 503 January 23, 2018 Oakville, Ontario L6H 5R7 Canada Submission Control No: 205852 Page 1 of 35 RECENT MAJOR LABEL CHANGES Not Applicable TABLE OF CONTENTS RECENT MAJOR LABEL CHANGES ......................................................................................2 TABLE OF CONTENTS ..............................................................................................................2 PART I: HEALTH PROFESSIONAL INFORMATION ............................................................4 1 INDICATIONS....................................................................................................................4 1.1 Pediatrics ....................................................................................................................4 1.2 Geriatrics ....................................................................................................................4 2 CONTRAINDICATIONS ..................................................................................................4 3 DOSAGE AND ADMINISTRATION ...............................................................................4 3.1 Dosing Considerations -

LONSURF® (Trifluridine and Tipiracil) Tablets Are a Prescription Medicine Used to Treat People with Colon Or Rectal Cancer That
LONSURF® (trifl uridine and tipiracil) tablets are a prescription medicine used to treat people with colon or rectal cancer that has spread to other parts of the body and who have been previously treated with or cannot receive certain chemotherapy medicines. It is not known if LONSURF is safe and effective in children. In a clinical trial, half of the patients treated with LONSURF were still alive at 7.1 months and half of the patients who received placebo were still alive at 5.3 months. Worsening of the disease or death occurred in 88% of patients treated with LONSURF and 94% of patients who received placebo. Selected Important Safety Information Low blood counts. LONSURF can decrease the number of your blood cells. This can sometimes be severe and life-threatening. Please see Important Safety Information on pages 18 and 19 and full Prescribing Information in pocket. Table of contents 4 About this booklet Support, tools, and resources 5 About colon or rectal cancer 34 Taiho Oncology Patient Support 35 LONSURF Starter Kit About LONSURF® (trifl uridine and tipiracil) tablets 36 Online resources 8 What is it? 37 Advocacy groups 9 Who is it for? 10 How it works Living with and managing colon or rectal cancer 11 What to expect 40 Coping with advanced colon or rectal cancer 14 How to take it 42 Tips for your appointments 44 Tips for your caregivers Safety 18 Important Safety Information Appendix 20 Managing side effects 48 Glossary 31 Potential treatment changes 54 Journal 60 Important contact information Please see Important Safety Information on pages 18 and 19 and full Prescribing Information in pocket. -

These Highlights Do Not Include All the Information Needed to Use LONSURF Safely and Effectively
LONSURF- trifluridine and tipiracil tablet, film coated Taiho Pharmaceutical Co., Ltd. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LONSURF safely and effectively. See full prescribing information for LONSURF. LONSURF (trifluridine and tipiracil) tablets, for oral use Initial U.S. Approval: 2015 RECENT MAJOR CHANGES Indications and Usage (1.2) 2/2019 Recommended Dosage (2.1) 2/2019 Warnings and Precaution (5.1) 2/2019 INDICATIONS AND USAGE LONSURF is a combination of trifluridine, a nucleoside metabolic inhibitor, and tipiracil, a thymidine phosphorylase inhibitor, indicated for the treatment of adult patients with: metastatic colorectal cancer who have been previously treated with fluoropyrimidine-, oxaliplatin- and irinotecan-based chemotherapy, an anti-VEGF biological therapy, and if RAS wild-type, an anti-EGFR therapy. (1.1) metastatic gastric or gastroesophageal junction adenocarcinoma previously treated with at least two prior lines of chemotherapy that included a fluoropyrimidine, a platinum, either a taxane or irinotecan, and if appropriate, HER2/neu- targeted therapy. (1.2) DOSAGE AND ADMINISTRATION Recommended Dosage: 35 mg/m2/dose orally twice daily with food on Days 1 through 5 and Days 8 through 12 of each 28-day cycle. (2.1) DOSAGE FORMS AND STRENGTHS Tablets: 15 mg trifluridine/6.14 mg tipiracil (3) 20 mg trifluridine/8.19 mg tipiracil (3) CONTRAINDICATIONS None. (4) WARNINGS AND PRECAUTIONS Severe Myelosuppression: Obtain complete blood counts prior to and on Day 15 of each cycle. Withhold and resume at next lower LONSURF dosage as recommended. (2.1, 5.1) Embryo-Fetal Toxicity: Can cause fetal harm. -

Patient Assistance Brochure | LONSURF® (Trifluridine and Tipiracil)
Important Safety Information LONSURF® (trifl uridine and tipiracil) may cause serious side effects, including: • Low blood counts. Low blood counts are common with LONSURF and can sometimes be severe and life-threatening. LONSURF can cause a decrease in your white blood cells, red blood cells, and platelets. Low white blood cells can make you more likely to get serious infections that could lead to death. Your healthcare provider should do blood tests before you receive LONSURF, at day 15 during treatment with LONSURF, and as needed to check your blood cell counts. Your healthcare provider may lower your dose of LONSURF or stop LONSURF if you have low white blood cell or platelet counts Tell your healthcare provider right away if you get any of the following signs and symptoms of infection during treatment with LONSURF: fever, chills, or body aches. Before taking LONSURF, tell your healthcare provider about all of your medical conditions, including if you: • Have kidney problems • Are pregnant or plan to become pregnant. LONSURF can harm your unborn baby – Females who can become pregnant should use effective birth control during treatment with LONSURF. Tell your healthcare provider immediately if you become pregnant – Males , while on treatment and for 3 months after your last dose of LONSURF, you should use a condom during sex with female partners who are able to become pregnant. Tell your healthcare provider right away if your partner becomes pregnant while you are taking LONSURF • Are breast-feeding or plan to breast-feed. It is not known if LONSURF passes into your breast milk. -

Aqueous Immiscible Layered Double Hydroxides – AIM-Ldhs Kanittika Ruengkajorn, Christopher M
Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is © the Partner Organisations 2018 Aqueous Immiscible Layered Double Hydroxides – AIM-LDHs Kanittika Ruengkajorn, Christopher M. R. Wright. Nicholas H. Rees, Jean-Charles Buffet and Dermot O’Hare* Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, OX3 1TA, Oxford. E-mail: [email protected] Table of contents 1. General experimental details S2 1.1. Analytical techniques for LDH characterisation S2 1.2. Synthesis of Conventional, AMO- and AIM-LDHs S4 2. Supplementary experimental data S6 2.1 Figures S6 2.2 Tables S36 3 References S47 S1 1. General experimental details 1.1 Analytical techniques for LDH characterisation 1.1.1. X-ray powder diffraction (XRD) X-ray powder diffraction (XRD) results were investigated by using a PANalytical X’Pert Pro diffractometer in reflection mode operating at a voltage of 40 kV and a current intensity of 40 mA with Cu-Kα radiation (λ = 1.5406 Å). LDHs and LDOs powder were placed into stainless steel sample holders. A 1° slit was used. Bragg reflections due to sample holder were observed at 2θ = 43-44° and 50° and from silicon wafer were located at 2θ = 33°, 62° and 69°. The Scherrer’s equation was used to estimate the mean crystallite domain length (CDL) of the LDHs; CDL = Kλ(βcosθ)-1, where CDL = the mean crystallite domain length, K = Scherrer constant, λ = the wavelength of the radiation, and β = the full-width at half-maximum height (FWHM) values of a reflection located at 2θ. Thus, the CDL along c- axis (CDL003) can be calculated from the full-width at half-maximum height values of the (003) Bragg reflection, which is assumed to be the total crystal thickness along the c-axis. -

Justification Document for the Selection of a Corap Substance
JUSTIFICATION DOCUMENT FOR THE SELECTION OF A CORAP SUBSTANCE ________________________________________________________________ Justification Document for the Selection of a CoRAP Substance Substance Name (public name): Diethyl Ether EC Number: 200-467-2 CAS Number: 60-29-7 Authority: France Date: 21/03/2017 Cover Note This document has been prepared by the evaluating Member State given in the CoRAP update. JUSTIFICATION DOCUMENT FOR THE SELECTION OF A CORAP SUBSTANCE _______________________________________________________________ Table of Contents 1 IDENTITY OF THE SUBSTANCE 3 1.1 Other identifiers of the substance 3 2 OVERVIEW OF OTHER PROCESSES / EU LEGISLATION 5 3 HAZARD INFORMATION (INCLUDING CLASSIFICATION) 7 3.1 Classification 7 3.1.1 Harmonised Classification in Annex VI of the CLP 7 3.1.2 Self classification 7 3.1.3 Proposal for Harmonised Classification in Annex VI of the CLP 7 4 INFORMATION ON (AGGREGATED) TONNAGE AND USES 8 4.1 Tonnage and registration status 8 4.2 Overview of uses 8 5. JUSTIFICATION FOR THE SELECTION OF THE CANDIDATE CORAP SUBSTANCE 10 5.1. Legal basis for the proposal 10 5.2. Selection criteria met (why the substance qualifies for being in CoRAP) 10 5.3 Initial grounds for concern to be clarified under Substance Evaluation 10 5.4 Preliminary indication of information that may need to be requested to clarify the concern 11 5.5 Potential follow-up and link to risk management 11 EC no 200-467-2 MSCA - France Page 2 of 11 JUSTIFICATION DOCUMENT FOR THE SELECTION OF A CORAP SUBSTANCE _______________________________________________________________ -

Lonsurf, INN-Trifluridine / Tipiracil
25 February 2016 EMA/CHMP/287846/2016 Committee for Medicinal Products for Human Use (CHMP) Assessment report Lonsurf International non-proprietary name: trifluridine / tipiracil Procedure No. EMEA/H/C/003897/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5520 Send a question via our website www.ema.europa.eu/contact An agency of the European Union Table of contents 1. Background information on the procedure .............................................. 7 1.1. Submission of the dossier ..................................................................................... 7 1.2. Steps taken for the assessment of the product ........................................................ 8 2. Scientific discussion ................................................................................ 9 2.1. Introduction ........................................................................................................ 9 2.2. Quality aspects .................................................................................................. 12 2.2.1. Introduction.................................................................................................... 12 2.2.2. Active substance ............................................................................................ 13 Trifluridine .............................................................................................................. -

Study Protocol and Statistical Analysis Plan
MC1642 3 Table of Contents Phase II Trial of FTD/TPI (TAS-102) in Biliary Tract Cancers ................................................................... 1 Protocol Resources ........................................................................................................................................ 2 Table of Contents .......................................................................................................................................... 3 Schema .......................................................................................................................................................... 4 1.0 Background ................................................................................................................................... 5 2.0 Goals ............................................................................................................................................. 8 3.0 Patient Eligibility .......................................................................................................................... 9 4.0 Study Schedules .......................................................................................................................... 11 5.0 Stratification Factors OR Grouping Factor: ................................................................................ 12 6.0 Registration Procedures .............................................................................................................. 12 7.0 Protocol Treatment ..................................................................................................................... -

207981Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 207981Orig1s000 PHARMACOLOGY REVIEW(S) MEMORANDUM Lonsurf (trifluridine and tipiracil) Date: September 18, 2015 To: File for NDA 207981 From: John K. Leighton, PhD, DABT Director, Division of Hematology Oncology Toxicology Office of Hematology and Oncology Products I have examined pharmacology/toxicology supporting and labeling reviews for Lonsurf conducted by Drs. Khasar and Fox, and secondary memorandum and labeling provided by Dr. Helms. Following finalization of the primary and secondary reviews, additional discussion occurred about the proper description of the established pharmacological class (EPC) for this combination product. As the anticancer activity of the drug is through the action of the trifluridine portion of the drug, the original recommendation was to use the EPC for this portion of the drug to describe the EPC for LONSURF. Upon further review and discussion, and consistent with practices with other combination products in which one component of the product is present primarily to affect the pharmacokinetics of the other component, the Division revised the recommendation for the EPC for LONSURF to be “LONSURF is a combination of trifluridine, a nucleoside metabolic inhibitor, and tipiracil, a thymidine phosphorylase inhibitor, indicated for…” I concur with Dr. Helms’ conclusion that Lonsurf may be approved for the proposed indication and that no additional nonclinical studies are needed. Reference ID: 3821960 --------------------------------------------------------------------------------------------------------- This is a representation of an electronic record that was signed electronically and this page is the manifestation of the electronic signature. --------------------------------------------------------------------------------------------------------- /s/ ---------------------------------------------------- JOHN K LEIGHTON 09/18/2015 Reference ID: 3821960 MEMORANDUM Date: August 25 , 2015 From: Whitney S. -

GRAS Notice 695, Dimethyl Ether
GRAS Notice (GRN) No. 695 http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm Callaghan Sl ~ TECHNOLOGY..,u CESS March 17, 2017 Dr. Paulette Gaynor Office of Food Additive Safety (FHS-200) MAR 2 0 2017 Center for Food Safety and Applied Nutrition Food and Drug Administration OFFICE OF FOOD ADDITIVE SAFETY 5100 Campus Drive College Park, MD 20740 Re: GRAS Notice for Dimethyl Ether Dear Dr. Gaynor In accordance with 21 CFR §170 Subpart E consisting of §170.203 through 170.285, Callaghan Innovation hereby informs the United States Food and Drug Administration of the conclusion that the intended use of dimethyl ether as an extraction solvent in the processing of various food products is Generally Recognized as Safe (GRAS) as described in the enclosed notice, and thereby the intended use of dimethyl ether is not subject to the premarket approval requirements of the Federal Food, Drug, and Cosmetic Act. I hereby certify that the enclosed electronic files were scanned for viruses prior to submission and are thus certified as being virus-free using McAfee VirusScan 8.8. Should you have any questions or concerns regarding this GRAS Notice, please do not hesitate to contact me at any point during the review process so that we may provide a response in a timely manner. Yours sincerely (b) (6) Stephen Tallon, Ph.D. Senior Research Engineer Callaghan Innovation GRAS Notice for the Use of Dimethyl Ether as an Extraction Solvent Prepared for: Office of Food Additive Safety (FHS-200) Center for Food Safety and Applied Nutrition Food and Drug Administration 5100 Campus Drive College Park, MD 20740 Submitted by: Callaghan Innovation 69 Gracefield Road Lower Hutt 5040 New Zealand March 7, 2017 GRAS Notice for the Use of Dimethyl Ether as an Extraction Solvent Table of Contents Page Part 1. -

TAS-102 for Treatment of Advanced Colorectal Cancers That Are No Longer Responding to Other Therapies
Author Manuscript Published OnlineFirst on April 28, 2016; DOI: 10.1158/1078-0432.CCR-15-2783 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. TAS-102 for Treatment of Advanced Colorectal Cancers that Are No Longer Responding to Other Therapies Daphne L. van der Velden1, Frans L. Opdam2, and Emile E. Voest1 1 The Netherlands Cancer Institute - Antoni van Leeuwenhoek Hospital, Department of Molecular Oncology, Amsterdam, the Netherlands 2 The Netherlands Cancer Institute - Antoni van Leeuwenhoek Hospital, Department of Clinical Pharmacology, Amsterdam, the Netherlands Corresponding Author: Frans L. Opdam, Division of Medical Oncology, The Netherlands Cancer Institute - Antoni van Leeuwenhoek Hospital, Postbus 90203, 1006 BE Amsterdam, the Netherlands. E-mail: [email protected] Running Title: TAS-102 for Treatment of Advanced Colorectal Cancer Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed. Abstract TAS-102 is a novel oral formulation of trifluridine (TFT) and tipiracil hydrochloride (TPI), a thymidine phosphorylase inhibitor. TFT was originally synthesized in the 1960s and is a nucleoside analog that impedes DNA synthesis by inhibition of thymidylate synthase. TFT’s main mechanism of action however seems to be its incorporation into DNA, which distinguishes TFT from current well-known antimetabolites like 5-fluorouracil (5-FU). The rapid degradation of TFT brought initial clinical development to a halt, but TFT re-entered clinical trials when addition of a TPI was found to improve the bio-availability of TFT. The combined TFT-TPI formulation was tested in patients with treatment- refractory metastatic colorectal cancer in the randomized phase III RECOURSE study. -

Phase I Study of Trifluridine/Tipiracil Plus Irinotecan and Bevacizumab in Advanced Gastrointestinal Tumors
Published OnlineFirst January 10, 2020; DOI: 10.1158/1078-0432.CCR-19-2743 CLINICAL CANCER RESEARCH | CLINICAL TRIALS: TARGETED THERAPY Phase I Study of Trifluridine/Tipiracil Plus Irinotecan and Bevacizumab in Advanced Gastrointestinal Tumors A C Anna M. Varghese1, Dana B. Cardin2, Jonathan Hersch1, Al B. Benson3, Howard S. Hochster4, Lukas Makris5, Kensuke Hamada6, Jordan D. Berlin2, and Leonard B. Saltz1 ABSTRACT ◥ Purpose: This two-part phase Ib trial determined the maximum toxicities (fatigue and neutropenia) were observed in the dose- tolerated dose (MTD) of the combination of trifluridine/tipiracil escalation phase, and MTD was defined as FTD/TPI 25 mg/m2 (FTD/TPI) and irinotecan in patients with advanced gastrointes- twice daily plus irinotecan 180 mg/m2.Intheexpansionphase, tinal tumors, and evaluated the safety, pharmacokinetics, and 83% (20/24) experienced any-cause grade 3 adverse events antitumor activity of the FTD/TPI, irinotecan, and bevacizumab (AEs) with the triplet combination, most frequently neutropenia triplet combination in previously treated metastatic colorectal (42%), leukopenia (25%), and diarrhea (12%). AEs of any-cause cancer (mCRC). led to dosing interruptions, modifications, and discontinuations Patients and Methods: Dose escalation (3þ3 design) in advanced in 29%, 17%, and 4% of patients, respectively. No treatment- gastrointestinal tumors was followed by expansion in mCRC. related deaths occurred. Three patients (12%) experienced partial During dose escalation, patients received FTD/TPI (20–35 mg/m2 responses and 16 (67%) patients had stable disease lasting twice daily; days 1–5 of a 14-day cycle) and irinotecan (120– >4 months. The median progression-free survival was 7.9 months 180 mg/m2; day 1).