The Impact on Our Health System New Clinical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
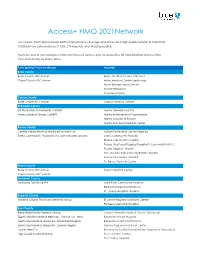
Access+ HMO 2021Network
Access+ HMO 2021Network Our Access+ HMO plan provides both comprehensive coverage and access to a high-quality network of more than 10,000 primary care physicians (PCPs), 270 hospitals, and 34,000 specialists. You have zero or low copayments for most covered services, plus no deductible for hospitalization or preventive care and virtually no claims forms. Participating Physician Groups Hospitals Butte County Butte County BSC Admin Enloe Medical Center Cohasset Glenn County BSC Admin Enloe Medical Center Esplanade Enloe Rehabilitation Center Orchard Hospital Oroville Hospital Colusa County Butte County BSC Admin Colusa Medical Center El Dorado County Hill Physicians Sacramento CalPERS Mercy General Hospital Mercy Medical Group CalPERS Methodist Hospital of Sacramento Mercy Hospital of Folsom Mercy San Juan Medical Center Fresno County Central Valley Medical Medical Providers Inc. Adventist Medical Center Reedley Sante Community Physicians Inc. Sante Health Systems Clovis Community Hospital Fresno Community Hospital Fresno Heart and Surgical Hospital A Community RMCC Fresno Surgical Hospital San Joaquin Valley Rehabilitation Hospital Selma Community Hospital St. Agnes Medical Center Glenn County Butte County BSC Admin Glenn Medical Center Glenn County BSC Admin Humboldt County Humboldt Del Norte IPA Mad River Community Hospital Redwood Memorial Hospital St. Joseph Hospital - Eureka Imperial County Imperial County Physicians Medical Group El Centro Regional Medical Center Pioneers Memorial Hospital Kern County Bakersfield Family Medical -

October 2, 2020 COVID-19 Update to UC Regents
January 8, 2021 Update COVID-19 AND 'CORONAVIRUS' UPDATES CARRIE L. BYINGTON, MD Executive Vice President, University of California Health THE IMPACT ON OUR HEALTH SYSTEM This is the 27th update for Regents regarding the SARS-CoV-2 virus pandemic and its impact on the University's health and academic enterprise. We usher in 2021 against a backdrop of failed national leadership and chaos in Washington DC. In this environment, the pandemic has been relentless. We are in the midst of the most severe surge experienced to date, with December 2020 the deadliest month of the pandemic thus far. I am very concerned that the post-Christmas, post- New Year's "surge on top of a surge," as described by Dr. Anthony Fauci of the National Institutes of Health, will lead to even worse outcomes and an even higher death toll this month. At the same time, we have given the first COVID-19 vaccine dose to ~ 85% of frontline health care workers across University of California Health (UCH), and expect all remaining priority 1A personnel who want to be vaccinated will receive their first dose by early next week. Some health care workers have already begun receiving their second doses. We look forward to having a fully immunized health workforce by February. COVID-19 BY THE NUMBERS COVID-19 cases and hospitalizations have soared since my last update on December 4. Nationally, the number of cases now exceeds 21.2 million, and the cumulative death toll stands at 359,849, according to data from the Centers for Disease Control and Prevention (CDC). -

Our University Innovation Is Central to Who We Are and What We Do at the University of California, San Diego
Our University Innovation is central to who we are and what we do at the University of California, San Diego. Here, students learn that knowledge isn’t just acquired in the classroom—life is their laboratory. UC San Diego is an academic powerhouse and economic engine, recognized as one of the top 10 public universities by U.S. News & World Report and ranked number one in the nation for public service by the Washington Monthly. Our location is unparalleled, our impact unmistakable. UC San Diego shapes minds, changes lives, launches industries and builds the future … one student, one discovery and one achievement at a time. Points of Distinction Scripps Institution of Oceanography climate scientist Charles David Keeling was the first to confirm the buildup of carbon dioxide in the atmosphere. His precise measurements, which he began calculating in 1958, produced a data set now known widely as the “Keeling Curve,” a benchmark of global warming studies. The Jacobs School of Engineering is home to the world’s first full-scale outdoor shake table, designed to create realistic simulations of the most devastating earthquakes on record to advance seismic safety. UC San Diego is unique among other UC campuses—our university offers undergraduates the “small college” concept patterned after those at Cambridge and Oxford. Each of the six undergraduate colleges has its own residence halls, student services, traditions and even graduation ceremonies. While the undergraduates remain part of one university, they also develop a sense of identity within the smaller family of their chosen college. In 1986 UC San Diego established the first Cognitive Science Department in the world, which has become one of the leading centers of this field. -
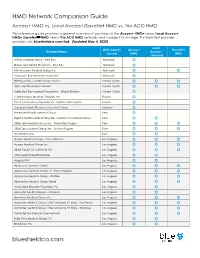
Blue Shield Comparison HMO Networks
HMO Network Comparison Guide Access+ HMO vs. Local Access+/SaveNet HMO vs. Trio ACO HMO This reference guide provides a general overview of providers in the Access+ HMO® versus Local Access+ HMO® /SaveNet℠ HMO versus Trio ACO HMO networks and is subject to change. For the latest provider updates, visit blueshieldca.com/fad. (Updated May 4, 2020) Local Main County Access+ Trio ACO Provider Name Access+ Served HMO HMO /Savenet Affinity Medical Group - East Bay Alameda O Brown And Toland Physicians - East Bay Alameda O Hill Physicians Medical Group Inc. Alameda O O Sutter East Bay Medical Foundation Alameda O Hill Physicians - Contra Costa County Contra Costa O O O John Muir Physicians Network Contra Costa O O O Sutter East Bay Medical Foundation - Diablo Division Contra Costa O Central Valley Medical Providers Inc. Fresno O Santé Community Physicians Inc. Santé Health System Fresno O Imperial County Physicians Medical Group Imperial O Bakersfield Family Medical Group Kern O Dignity Health Medical Network - Central CA Medical Group Kern O O GEMCare Medical Group Inc. - Bakersfield Region Kern O O O GEMCare Medical Group Inc. - Delano Region Kern O O O Health Now IPA Kern O O Access Medical Group - Santa Monica Los Angeles O O O Access Medical Group Inc. Los Angeles O O O Allied Pacific Of California IPA Los Angeles O O O Alta Medical Health Services Los Angeles O O Angeles IPA Los Angeles O O Applecare Medical Group Los Angeles O O O Applecare Medical Group - St. Francis Region Los Angeles O O O Applecare Medical Group - Whittier Los Angeles O O O Applecare Medical Group Select Los Angeles O O O Associated Hispanic Physicians IPA Los Angeles O O Axminster Medical Group - Olympia Los Angeles O O Axminster Medical Group Inc. -

RETURN to LEARN STAFF TOWN HALL Tuesday, July 20, 2021 TODAY’S TOWN HALL IS HOSTED BY
RETURN TO LEARN STAFF TOWN HALL Tuesday, July 20, 2021 TODAY’S TOWN HALL IS HOSTED BY UC San Diego Executive Vice Chancellor Elizabeth H. Simmons Opening Remarks Chief Human Resources Officer Nancy E. Resnick Host Strategic Communications and Engagement Manager for Campus Human Resources Hallie Nicholson Panel Moderator TODAY’S TOWN HALL WILL INCLUDE THE FOLLOWING PANELISTS • Stephen Jackson, Associate Vice • Nancy Resnick, Chief Human Chancellor, Resource Management & Resources Officer Planning • Angela Scioscia, M.D., Interim Director, • Bryan McNutt, Ph.D., Faculty & Staff Student Health & Well-Being Services Assistance Program Counselor • Chip Schooley, M.D., Professor of • Erik Mitchell, Ph.D., University Librarian Medicine • Pierre Ouillet, Vice Chancellor & CFO • Terri Winbush, Senior Director of Labor Relations and Employee Relations CAMPUS SAFETY PROTOCOLS • Regardless of vaccination status, UC San Diego employees working on campus must continue to mask indoors. • The safety of our campus community will guide any adjustments to campus safety protocols or operations. • Updated guidance on the proposed UC COVID Vaccine Mandate is expected from the UC Office of the President by the end of July. • Stay up to date by visiting returntolearn.ucsd.edu RETURNING TO CAMPUS Our return to campus is being guided by: • Safety and equity • Operational needs of the university • Flexibility and support Advance notice from your VC or supervisor will be provided for any changes in current work arrangements. Resources are available to support your successful return to campus. Vaccines are crucial to the health and well-being of our entire campus community. Appointments are available at the Price Center COVID-19 Vaccination Site. -

UC SAN DIEGO PROJECT SUMMARIES 2017-18 and 2018-19 Health
UC SAN DIEGO PROJECT SUMMARIES 2017-18 and 2018-19 Health Hillcrest Outpatient Facility • Provide approximately 250,000 GSF • Deliver a new Outpatient Pavilion and medical office building of approximately 250,000 gross square feet on the Hillcrest medical center campus. Project would include a cancer care center and infusion clinic. • Provide outpatient surgical and primary care facilities that serve the urban San Diego communities. La Jolla, Perlman Medical Offices, Interior Renovations • Perlman Medical Offices, a multispecialty clinic building constructed on the La Jolla Campus in 1993, requires moderate renovations on the first and second floors and cosmetic upgrades on the third floor to meet changing clinic needs and patient expectations. • Renovations would be conducted in stages. • Retrofits and/or replacement of major HVAC components, to provide a safe and comfortable environment for building occupants. • ADA improvements to be compliant with current code. La Jolla, Thornton Hospital Geriatric Emergency Department and Radiology Intake Center (RIC) • The proposed project would renovate approximately 12,300 gross square feet of existing tenant improvement space (lab and offices), on the ground floor at Thornton Hospital, to create a new Geriatric Emergency Department (ED) and Radiology Intake Center (RIC). The ED and RIC would accommodate increased patient loads due to the recent completion of the 245-bed Jacobs Medical Center (JMC) tower. La Jolla, Thornton Renovations 2: East Entry and Imaging Expansion • This project proposes to renovate approximately 19,000 gross square feet encompassing the current East Entry and Atrium at Thornton Hospital. The scope of work includes the demolition and redesign of the existing entry and adjacent East façade, and a complete refresh of three levels of interior atrium space. -

Points of Distinction
Points of Distinction • Arts and Humanities Home to a top-ranked department of theatre and dance, as well as a cutting-edge music department with one of the acoustically finest small concert halls in the world, the Division of Arts and Humanities invites the community to participate in the making of tomorrow’s culture. Two Pulitzer Prize winners are among the many decorated members of the faculty. UC San Diego’s preeminent Stuart Collection of site-specific sculpture tops off the unique offerings. • Biological Sciences Direct evidence of how a cancer gene works was achieved by UC San Diego biomedical researchers, including biology professor Russell Doolittle, who were the first to show that a particular oncogene (gene known to cause cancer) matched a normal growth factor; the study appeared in Science in 1984. • Extended Studies and Public Programs (Extension) In 1985 Extension took the leadership role in creating CONNECT, the first innovation-business accelerator of its kind, which since then has assisted in the formation of 3,000 companies in the San Diego region. The highly praised program has been modeled in more than 50 regions around the world. In 2006 to broaden its mandate to include public advocacy, CONNECT spun out to become a trade association with Extension as a member and sponsor. • Health—Clinical Surgeons at UC San Diego Health have performed more pulmonary thromboendarterectomies (PTE) – a lifesaving surgery to clear the lung’s arteries of scar like tissue that robs patients of their ability to breathe – than any other institution in the world. Pioneered in 1970 by Kenneth Moser and Stuart Jamieson, the UC San Diego PTE program has a mortality rate of less than 1 percent for over 3,000 surgeries to date, the lowest known postoperative mortality rate worldwide. -

UC San Diego Health Patients, As Well As Patients Seen Throughout UC Health System
Testimony of Dr. Charles Daniels House Energy and Commerce Committee 340B Oversight Hearing, July 11, 2018 Introduction Good morning, Chairman Burgess and Chairman Walden, Ranking Member Green, and Ranking Member Pallone. Thank you for this opportunity to share my experience with the 340B Drug Pricing Program. I want to also want to say hello to Congressman Peters, my own Congressman, who serves on this Committee, along with Congresswoman Matsui, who represents the people of our sister institution UC Davis Health. I have been able to personally share with Congressman Peters and Congresswoman Matsui the value of the 340B discount to UC San Diego Health patients, as well as patients seen throughout UC Health System. My name is Dr. Charles Daniels, and I serve as Pharmacist-In-Chief for the University of California San Diego’s academic medical center, referred to as UC San Diego Health. As Pharmacist-In-Chief, I oversee UC San Diego Health’s administration and use of the 340B Program. Who is UC San Diego Health? UC San Diego Health is a public academic medical center serving the people of San Diego and surrounding communities. The medical center’s service imprint extends over 100 miles into remote El Centro in Imperial County. Our mission is to deliver outstanding patient care through commitment to the community, groundbreaking research, and inspired teaching. UC San Diego Health, a premier provider of tertiary and quaternary services, is comprised of three major inpatient facilities, the Hillcrest Medical Center, the Jacobs Medical Center, and the Sulpizio Cardiovascular Center, along with the region’s only National Cancer Institute (NCI)-designated Comprehensive Cancer Center. -

Technology and Partnerships Will Bring Expertise Closer to Home by Andrew N
AMERICAN HOSPITALS REPORT 2018 Future Trends in U.S. Medical Innovation: Technology and Partnerships Will Bring Expertise Closer to Home By Andrew N. Garman, PsyD, Executive Director, U.S. Cooperative for International Patient Programs (USCIPP); CEO, National Center for Healthcare Leadership; Professor, Department of Health Systems Management, Rush University; Tricia Johnson, PhD, Research Director, USCIPP; Professor and Associate Chair of Research & Education, Department of Health Systems Management, Rush University; Director, Rush Center for the Advancement of Healthcare Value; Jarrett Fowler, MPPA, Senior Manager, USCIPP; Callie Lambert, Research Manager, USCIPP he U.S. has a longstanding reputation to continue expanding disproportionately to over time it will increasingly take the form for advancing the science necessary overall population growth. of incentives for consumers to get their care to develop and sustain medical As average life expectancy increases, the from providers who can demonstrate they are breakthroughs. Over many decades, ratio of working-age adults to retirement- delivering better outcomes at lower costs. Tsubstantial investments from the National age adults will continue to shrink. The ratio Institutes of Health as well as charitable of healthcare professionals to retirement Trend #4 - Medical innovation will foundations have led American academic age adults will also continue to decline, and accelerate – and costs along with it medical centers and health systems to become health systems will need to find ways to The group of innovations collectively known hubs of medical innovation, attracting patients evolve their care delivery models to address as ‘precision medicine’ holds promise for rapid from many countries who travel for advanced these greater needs with fewer people. -

2019 Capital Financial Plan
Attachment 1 Capital Financial Plan 2019-25 University of California Office of the President Capital Asset Strategies & Finance 1111 Franklin Street, 6th Floor Oakland, California 94607-5200 Cover photo: UC Berkeley Photo credit: Elena Zhukova 2019-25 CAPITAL FINANCIAL PLAN TABLE OF CONTENTS Summary 5 CAPITAL PLAN BY LOCATION How to Read the Tables 17 Berkeley 19 Davis 27 UC Davis Health 33 Irvine 39 UC Irvine Health 47 Los Angeles 53 UC Los Angeles Health 58 Merced 63 Riverside 69 San Diego 75 UC San Diego Health 83 San Francisco 89 UCSF Health 94 Santa Barbara 99 Santa Cruz 107 Division of Agriculture and Natural Resources 115 Lawrence Berkeley National Laboratory 119 Systemwide and Office of the President 125 Appendix – Projects of Interest to UC Health 130 2019-25 CAPITAL FINANCIAL PLAN 4 SUMMARY The University’s capital program is driven by the campuses’ and medical centers’ academic and strategic plans. The Capital Financial Plan (CFP) is developed based on the needs at each location for buildings and other physical infrastructure to achieve these overarching plans. ▪ Strategic and Academic Plans define priority areas and goals and may include institutional aspirations. ▪ The Long Range Development Plan is a comprehensive plan, as approved by the Regents, on proposed future physical planning and development of a campus or medical center. ▪ The Physical Design Framework identifies planning principles and objectives for design of the physical environment. The CFP presents proposed capital projects, public private partnerships, and acquisition of real property that support these plans. The 2019-25 CFP represents $52 billion of capital need as articulated by the campuses and medical centers over this year and the next five fiscal years (through 2024-25). -
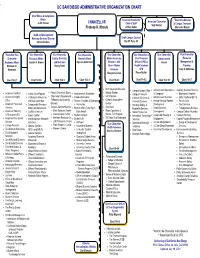
Uc San Diego Administrative Organization Chart
UC SAN DIEGO ADMINISTRATIVE ORGANIZATION CHART Chief Ethics & Compliance Officer Associate Chancellor **Executive Director Associate Chancellor Judith Bruner CHANCELLOR Chief of Staff & Campus Treasurer Suzi Sterner Pradeep K. Khosla Jeffrey Gattas Mercedes Munoz Audit and Management Advisory Services Director Chief Campus Counsel Christa Perkins Dan W. Park, JD *Executive Vice Vice Chancellor Vice Chancellor Vice Chancellor Vice Chancellor Vice Chancellor & Vice Chancellor Vice Chancellor Chancellor Research Affairs Equity, Diversity, Student Affairs Marine Sciences, Chief Financial Advancement Resource Academic Affairs Sandra A. Brown and Inclusion Alysson Satterlund Director – SIO, Officer (CFO) & Vacant Management & Elizabeth H. Becky Petitt Dean – Marine Health Sciences Planning Simmons Sciences CFO Gary C. Matthews Margaret Leinen Pierre Ouillet Chart 10-0.1 Chart 10-0.6 Chart 10-0.3 Chart 10-0.8 Chart 10-0.5 Chart 10-0.2 Chart 10-0.10 Chart 10-0.7 Birch Aquarium Museum Campus Budget Office Advancement Operations Auxiliary Business Services Faculty Diversity & Equity Biology Section Academic Facilities Animal Care Program Assessment & Evaluation Campus Treasurer & Campaign Bookstore & Imprints Chancellor’s Postdoctoral Earth Section Academic Integrity Institutional Animal Care Campus Recreation Controller/Business & Advancement Services Early Childhood Ed Ctr Fellowship for Academic Ocean & Atmosphere Office and Use Committee Finance, Facilities & Operations Financial Services Annual Giving & Pipeline Faculty -

Major Teaching Hospitals and Cancer Centers
Major Hospitals and Healthcare Groups with Cancer Centers Note: Those with a red * are NCI-designated centers. Those with a purple + are National Pancreas Foundation Pancreatic Cancer Centers. Alabama Birmingham Brookwood Baptist Health General information: 205-783-3000 or 877-909-4233 Website: https://www.brookwoodbaptisthealth.com/our-services/cancer University of Alabama Comprehensive Cancer Center* Cancer center phone: 800-UAB-0933 or 205-975-8222 Website: http://www3.ccc.uab.edu/ Alaska Anchorage Providence Alaska Medical Center Cancer center phone: 907-212-6870 Website: https://alaska.providence.org/services/c/cancer Arizona Phoenix Banner MD Anderson Cancer Center Cancer center phone: 480-256-644 Website: https://www.bannerhealth.com/banner-md-anderson Virginia C. Piper Cancer Center at Honor Health Cancer center phone: 480-323-1000 Website: https://www.honorhealth.com/locations/specialty-care/virginia-g-piper- cancer-center Tucson Banner – University Medical Center Tucson Cancer center phone: 520-694-0111 Website: https://www.bannerhealth.com/locations/tucson/banner-university- medical-center-tucson Arkansas Little Rock UAMS Winthrop P. Rockefeller Cancer Institute Cancer center phone: 501-296-1200 Website: https://cancer.uams.edu/ California Los Angeles area Cedars-Sinai Medical Center Cancer center phone: 800-CEDARS-1 Website: https://www.cedars-sinai.org/programs/cancer.html City of Hope* Cancer center phone: 800-826-4673 Website: https://www.cityofhope.org/homepage Loma Linda University Health – Cancer Center Cancer center