Interaction of Heavy Charged Particles with Matter BAEN-625 Advances in Food Engineering Heavy Charged Particles
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -
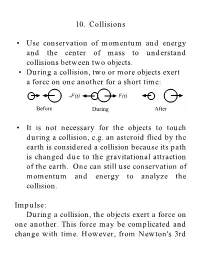
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Impulse and Momentum
Impulse and Momentum All particles with mass experience the effects of impulse and momentum. Momentum and inertia are similar concepts that describe an objects motion, however inertia describes an objects resistance to change in its velocity, and momentum refers to the magnitude and direction of it's motion. Momentum is an important parameter to consider in many situations such as braking in a car or playing a game of billiards. An object can experience both linear momentum and angular momentum. The nature of linear momentum will be explored in this module. This section will discuss momentum and impulse and the interconnection between them. We will explore how energy lost in an impact is accounted for and the relationship of momentum to collisions between two bodies. This section aims to provide a better understanding of the fundamental concept of momentum. Understanding Momentum Any body that is in motion has momentum. A force acting on a body will change its momentum. The momentum of a particle is defined as the product of the mass multiplied by the velocity of the motion. Let the variable represent momentum. ... Eq. (1) The Principle of Momentum Recall Newton's second law of motion. ... Eq. (2) This can be rewritten with accelleration as the derivate of velocity with respect to time. ... Eq. (3) If this is integrated from time to ... Eq. (4) Moving the initial momentum to the other side of the equation yields ... Eq. (5) Here, the integral in the equation is the impulse of the system; it is the force acting on the mass over a period of time to . -

Decays of the Tau Lepton*
SLAC - 292 UC - 34D (E) DECAYS OF THE TAU LEPTON* Patricia R. Burchat Stanford Linear Accelerator Center Stanford University Stanford, California 94305 February 1986 Prepared for the Department of Energy under contract number DE-AC03-76SF00515 Printed in the United States of America. Available from the National Techni- cal Information Service, U.S. Department of Commerce, 5285 Port Royal Road, Springfield, Virginia 22161. Price: Printed Copy A07, Microfiche AOl. JC Ph.D. Dissertation. Abstract Previous measurements of the branching fractions of the tau lepton result in a discrepancy between the inclusive branching fraction and the sum of the exclusive branching fractions to final states containing one charged particle. The sum of the exclusive branching fractions is significantly smaller than the inclusive branching fraction. In this analysis, the branching fractions for all the major decay modes are measured simultaneously with the sum of the branching fractions constrained to be one. The branching fractions are measured using an unbiased sample of tau decays, with little background, selected from 207 pb-l of data accumulated with the Mark II detector at the PEP e+e- storage ring. The sample is selected using the decay products of one member of the r+~- pair produced in e+e- annihilation to identify the event and then including the opposite member of the pair in the sample. The sample is divided into subgroups according to charged and neutral particle multiplicity, and charged particle identification. The branching fractions are simultaneously measured using an unfold technique and a maximum likelihood fit. The results of this analysis indicate that the discrepancy found in previous experiments is possibly due to two sources. -

Charged Particle Radiotherapy
Corporate Medical Policy Charged Particle Radiotherapy File Name: charged_particle_radiotherapy Origination: 3/12/96 Last CAP Review: 5/2021 Next CAP Review: 5/2022 Last Review: 5/2021 Description of Procedure or Service Cha rged-particle beams consisting of protons or helium ions or carbon ions are a type of particulate ra dia tion therapy (RT). They contrast with conventional electromagnetic (i.e., photon) ra diation therapy due to several unique properties including minimal scatter as particulate beams pass through tissue, and deposition of ionizing energy at precise depths (i.e., the Bragg peak). Thus, radiation exposure of surrounding normal tissues is minimized. The theoretical advantages of protons and other charged-particle beams may improve outcomes when the following conditions a pply: • Conventional treatment modalities do not provide adequate local tumor control; • Evidence shows that local tumor response depends on the dose of radiation delivered; and • Delivery of adequate radiation doses to the tumor is limited by the proximity of vital ra diosensitive tissues or structures. The use of proton or helium ion radiation therapy has been investigated in two general categories of tumors/abnormalities. However, advances in photon-based radiation therapy (RT) such as 3-D conformal RT, intensity-modulated RT (IMRT), a nd stereotactic body ra diotherapy (SBRT) a llow improved targeting of conventional therapy. 1. Tumors located near vital structures, such as intracranial lesions or lesions a long the a xial skeleton, such that complete surgical excision or adequate doses of conventional radiation therapy are impossible. These tumors/lesions include uveal melanomas, chordomas, and chondrosarcomas at the base of the skull and a long the axial skeleton. -

12. Elastic Collisions A) Overview B) Elastic Collisions V
12. Elastic Collisions A) Overview In this unit, our focus will be on elastic collisions, namely those collisions in which the only forces that act during the collision are conservative forces. In these collisions, the sum of the kinetic energies of the objects is conserved. We will find that the description of these collisions is significantly simplified in the center of mass frame of the colliding objects. In particular, we will discover that, in this frame, the speed of each object after the collision is the same as its speed before the collision. B) Elastic Collisions In the last unit, we discussed the important topic of momentum conservation. In particular, we found that when the sum of the external forces acting on a system of particles is zero, then the total momentum of the system, defined as the vector sum of the individual momenta, will be conserved. We also determined that the kinetic energy of the system, defined to be the sum of the individual kinetic energies, is not necessarily conserved in collisions. Whether or not this energy is conserved is determined by the details of the forces that the components of the system exert on each other. In the last unit, our focus was on inelastic collisions, those collisions in which the kinetic energy of the system was not conserved. In particular non-conservative work was done by the forces that the individual objects exerted on each other during the collision. In this unit, we will look at examples in which the only forces that act during the collision are conservative forces. -

Collisions in Classical Mechanics in Terms of Mass-Momentum “Vectors” with Galilean Transformations
World Journal of Mechanics, 2020, 10, 154-165 https://www.scirp.org/journal/wjm ISSN Online: 2160-0503 ISSN Print: 2160-049X Collisions in Classical Mechanics in Terms of Mass-Momentum “Vectors” with Galilean Transformations Akihiro Ogura Laboratory of Physics, Nihon University, Matsudo, Japan How to cite this paper: Ogura, A. (2020) Abstract Collisions in Classical Mechanics in Terms of Mass-Momentum “Vectors” with Galilean We present the usefulness of mass-momentum “vectors” to analyze the colli- Transformations. World Journal of Mechan- sion problems in classical mechanics for both one and two dimensions with ics, 10, 154-165. Galilean transformations. The Galilean transformations connect the mass- https://doi.org/10.4236/wjm.2020.1010011 momentum “vectors” in the center-of-mass and the laboratory systems. We Received: August 28, 2020 show that just moving the two systems to and fro, we obtain the final states in Accepted: October 9, 2020 the laboratory systems. This gives a simple way of obtaining them, in contrast Published: October 12, 2020 with the usual way in which we have to solve the simultaneous equations. For Copyright © 2020 by author(s) and one dimensional collision, the coefficient of restitution is introduced in the Scientific Research Publishing Inc. center-of-mass system. This clearly shows the meaning of the coefficient of This work is licensed under the Creative restitution. For two dimensional collisions, we only discuss the elastic colli- Commons Attribution International sion case. We also discuss the case of which the target particle is at rest before License (CC BY 4.0). http://creativecommons.org/licenses/by/4.0/ the collision. -

AIAA 19Th Fluid Dynamics, Plasma Dynamics and Lasers Conference June 8-10, 1987/Honolulu, Hawaii
AIAA-87 -1407 Electron-Cyclotron-Resonance (ECR) Plasma Acceleration J. C. Sercel Jet Propulsion Laboratory California Institute of Technology Pasadena, California AIAA 19th Fluid Dynamics, Plasma Dynamics and Lasers Conference June 8-10, 1987/Honolulu, Hawaii For permission to copy or republish, contact the American Institute of Aeronautics and Astronautics 1633 Broadway, New York, NY 10019 AIAA-87-1407 ELECTRON-CYCLOTRON-RESONANCE (ECR) PLASMA ACCELERATION Joel C. Sercel* Jet Propulsion Laboratory California Institute of Technology Pasadena, California Abstract P power per unit volume, W/m3 R position vector, m A research effort directed at analytically v velocity, mls and experimentally investigating Electron U energy, J or eV Cyclotron-Resonance (ECR) plasma acceleration V electrostatic potential, volts is outlined. Relevant past research is reviewed. T temperature, Kelvin or eV The prospects for application of ECR plasma acceleration to spacecraft propulsion are described. It is shown that previously unexplained losses in converting microwave magnetic dipole moment power to directed kinetic power via ECR plasma reaction cross section, m2 acceleration can be understood in terms of diffusion of energized plasma to the physical time constant, s walls of the accelerator. It is argued that line radiation losses from electron-ion and electron SubscriPts atom inelastic collisions should be less than estimated in past research. Based on this new A acceleration understanding, the expectation now exists that B Bohm efficient ECR plasma accelerators can be e electron designed for application to high specific impulse ex excitation spacecraft propulsion. ionization summation variable refers to lowest energy level Acronyms and Abbreviations p perpendicular r relative D-He3 Deuterium Helium-Three sp space charge induced ECR Electron-Cyclotron-Resonance to t total GE General Electric JPL Jet Propulsion Laboratory LeRC Lewis Research Center I. -

The Basic Interactions Between Photons and Charged Particles With
Outline Chapter 6 The Basic Interactions between • Photon interactions Photons and Charged Particles – Photoelectric effect – Compton scattering with Matter – Pair productions Radiation Dosimetry I – Coherent scattering • Charged particle interactions – Stopping power and range Text: H.E Johns and J.R. Cunningham, The – Bremsstrahlung interaction th physics of radiology, 4 ed. – Bragg peak http://www.utoledo.edu/med/depts/radther Photon interactions Photoelectric effect • Collision between a photon and an • With energy deposition atom results in ejection of a bound – Photoelectric effect electron – Compton scattering • The photon disappears and is replaced by an electron ejected from the atom • No energy deposition in classical Thomson treatment with kinetic energy KE = hν − Eb – Pair production (above the threshold of 1.02 MeV) • Highest probability if the photon – Photo-nuclear interactions for higher energies energy is just above the binding energy (above 10 MeV) of the electron (absorption edge) • Additional energy may be deposited • Without energy deposition locally by Auger electrons and/or – Coherent scattering Photoelectric mass attenuation coefficients fluorescence photons of lead and soft tissue as a function of photon energy. K and L-absorption edges are shown for lead Thomson scattering Photoelectric effect (classical treatment) • Electron tends to be ejected • Elastic scattering of photon (EM wave) on free electron o at 90 for low energy • Electron is accelerated by EM wave and radiates a wave photons, and approaching • No -

Definitions and Concepts for AQA Physics a Level
Definitions and Concepts for AQA Physics A Level Topic 4: Mechanics and Materials Breaking Stress: The maximum stress that an object can withstand before failure occurs. Brittle: A brittle object will show very little strain before reaching its breaking stress. Centre of Mass: The single point through which all the mass of an object can be said to act. Conservation of Energy: Energy cannot be created or destroyed - it can only be transferred into different forms. Conservation of Momentum: The total momentum of a system before an event, must be equal to the total momentum of the system after the event, assuming no external forces act. Couple: Two equal and opposite parallel forces that act on an object through different lines of action. It has the effect of causing a rotation without translation. Density: The mass per unit volume of a material. Efficiency: The ratio of useful output to total input for a given system. Elastic Behaviour: If a material deforms with elastic behaviour, it will return to its original shape when the deforming forces are removed. The object will not be permanently deformed. Elastic Collision: A collision in which the total kinetic energy of the system before the collision is equal to the total kinetic energy of the system after the collision. Elastic Limit: The force beyond which an object will no longer deform elastically, and instead deform plastically. Beyond the elastic limit, when the deforming forces are removed, the object will not return to its original shape. Elastic Strain Energy: The energy stored in an object when it is stretched. -

Charged Current Anti-Neutrino Interactions in the Nd280 Detector
CHARGED CURRENT ANTI-NEUTRINO INTERACTIONS IN THE ND280 DETECTOR BRYAN E. BARNHART HIGH ENERGY PHYSICS UNIVERSITY OF COLORADO AT BOULDER ADVISOR: ALYSIA MARINO Abstract. For the neutrino beamline oscillation experiment Tokai to Kamioka, the beam is clas- sified before oscillation by the near detector complex. The detector is used to measure the flux of different particles through the detector, and compare them to Monte Carlo Simulations. For this work, theν ¯µ background of the detector was isolated by examining the Monte Carlo simulation and determining cuts which removed unwanted particles. Then, a selection of the data from the near detector complex underwent the same cuts, and compared to the Monte Carlo to determine if the Monte Carlo represented the data distribution accurately. The data was found to be consistent with the Monte Carlo Simulation. Date: November 11, 2013. 1 Bryan E. Barnhart University of Colorado at Boulder Advisor: Alysia Marino Contents 1. The Standard Model and Neutrinos 4 1.1. Bosons 4 1.2. Fermions 5 1.3. Quarks and the Strong Force 5 1.4. Leptons and the Weak Force 6 1.5. Neutrino Oscillations 7 1.6. The Relative Neutrino Mass Scale 8 1.7. Neutrino Helicity and Anti-Neutrinos 9 2. The Tokai to Kamioka Experiment 9 2.1. Japan Proton Accelerator Research Complex 10 2.2. The Near Detector Complex 12 2.3. The Super-Kamiokande Detector 17 3. Isolation of the Anti-Neutrino Component of Neutrino Beam 19 3.1. Experiment details 19 3.2. Selection Cuts 20 4. Cut Descriptions 20 4.1. Beam Data Quality 20 4.2. -

Chapter 3. Fundamentals of Dosimetry
Chapter 3. Fundamentals of Dosimetry Slide series of 44 slides based on the Chapter authored by E. Yoshimura of the IAEA publication (ISBN 978-92-0-131010-1): Diagnostic Radiology Physics: A Handbook for Teachers and Students Objective: To familiarize students with quantities and units used for describing the interaction of ionizing radiation with matter Slide set prepared by E.Okuno (S. Paulo, Brazil, Institute of Physics of S. Paulo University) IAEA International Atomic Energy Agency Chapter 3. TABLE OF CONTENTS 3.1. Introduction 3.2. Quantities and units used for describing the interaction of ionizing radiation with matter 3.3. Charged particle equilibrium in dosimetry 3.4. Cavity theory 3.5. Practical dosimetry with ion chambers IAEA Diagnostic Radiology Physics: a Handbook for Teachers and Students – chapter 3, 2 3.1. INTRODUCTION Subject of dosimetry : determination of the energy imparted by radiation to matter. This energy is responsible for the effects that radiation causes in matter, for instance: • a rise in temperature • chemical or physical changes in the material properties • biological modifications Several of the changes produced in matter by radiation are proportional to absorbed dose , giving rise to the possibility of using the material as the sensitive part of a dosimeter There are simple relations between dosimetric and field description quantities IAEA Diagnostic Radiology Physics: a Handbook for Teachers and Students – chapter 3, 3 3.2. QUANTITIES AND UNITS USED FOR DESCRIBING THE INTERACTION OF IONIZING RADIATION