Relacorilant with Nab-Paclitaxel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNITED STATES SECURITIES and EXCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Date of Report: June 21, 2007 (Date of earliest event reported) Corcept Therapeutics Incorporated (Exact name of registrant as specified in its charter) CA 000-50679 77-0487658 (State or other jurisdiction (Commission File (IRS Employer of incorporation) Number) Identification Number) 149 Commonwealth Drive 94025 (Address of principal executive offices) (Zip Code) 650-327-3270 (Registrant's telephone number, including area code) Not Applicable (Former Name or Former Address, if changed since last report) Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions: o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Item 8.01. Other Events On June 21, 2007 Corcept Therapeutics Incorporated issued a press release announcing positive results from its proof of concept study evaluating the ability of CORLUX to mitigate weight gain associated with Olanzapine. Item 9.01. Financial Statements and Exhibits (a) Financial statements: None (b) Pro forma financial information: None (c) Shell company transactions: None (d) Exhibits 99.1 Press Release of Corcept Therapeutics Incorporated dated June 21, 2007 SIGNATURE Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized. -

CORCEPT THERAPEUTICS INCORPORATED (Exact Name of Corporation As Specified in Its Charter)
Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-Q x QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended March 31, 2014 or ¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number: 000-50679 CORCEPT THERAPEUTICS INCORPORATED (Exact Name of Corporation as Specified in Its Charter) Delaware 77-0487658 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 149 Commonwealth Drive Menlo Park, CA 94025 (Address of principal executive offices, including zip code) (650) 327-3270 (Registrant’s telephone number, including area code) Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨ Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨ Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. -

PRESCRIBING INFORMATION KORLYM® (Mifepristone) 300 Mg Tablets These Highlights Do Not Include All the Information Needed to Use KORLYM® Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION KORLYM® (mifepristone) 300 mg Tablets These highlights do not include all the information needed to use KORLYM® safely and effectively. See full prescribing information for KORLYM®. • Patients receiving systemic corticosteroids for lifesaving purposes (4) KORLYM® (mifepristone) 300 mg Tablets • Women with a history of unexplained vaginal bleeding or endometrial hyperplasia with atypia or endometrial carcinoma (4) Initial U.S. Approval 2000 • Patients with known hypersensitivity to mifepristone or to any of the product WARNING: TERMINATION OF PREGNANCY components (4) See full prescribing information for complete boxed warning. WARNINGS AND PRECAUTIONS Mifepristone has potent antiprogestational effects and will result in the termination • Adrenal insufficiency: Patients should be closely monitored for signs and symptoms of of pregnancy. Pregnancy must therefore be excluded before the initiation of adrenal insufficiency (5.1). treatment with KORLYM, or if treatment is interrupted for more than 14 days in • Hypokalemia : Hypokalemia should be corrected prior to treatment and monitored for females of reproductive potential. during treatment (5.2). • Vaginal bleeding and endometrial changes: Women may experience endometrial RECENT MAJOR CHANGES thickening or unexpected vaginal bleeding. Use with caution if patient also has a Dosage and Administration (2.5) 11/2019 hemorrhagic disorder or is on anti-coagulant therapy (5.3). • QT interval prolongation: Avoid use with QT interval-prolonging drugs, or in patients with INDICATIONS AND USAGE potassium channel variants resulting in a long QT interval (5.4). • Use of Strong CYP3A Inhibitors: Concomitant use can increase mifepristone plasma levels. KORLYM (mifepristone) is a cortisol receptor blocker indicated to control hyperglycemia Use only when necessary and limit mifepristone dose to 900 mg (5.6). -

Corcept Therapeutics Announces Third Quarter 2020 Financial Results and Provides Corporate Update
Corcept Therapeutics Announces Third Quarter 2020 Financial Results and Provides Corporate Update November 3, 2020 MENLO PARK, Calif., Nov. 03, 2020 (GLOBE NEWSWIRE) -- Corcept Therapeutics Incorporated (NASDAQ: CORT), a commercial-stage company engaged in the discovery and development of drugs to treat severe metabolic, oncologic and psychiatric disorders by modulating the effects of the stress hormone cortisol, today reported its results for the quarter ended September 30, 2020. Financial Highlights Revenue of $86.3 million, a 6 percent increase from third quarter 2019 GAAP diluted net income of $0.17 per share, compared to $0.22 per share in third quarter 2019 Non-GAAP diluted net income of $0.24 per share, compared to $0.31 per share in third quarter 2019 Cash and investments of $444.2 million, compared to $409.6 million at June 30, 2020 Announcement of $200 million stock repurchase program 2020 revenue guidance narrowed to $355 – 365 million Revenue was $86.3 million in the third quarter, compared to $81.5 million in the third quarter of 2019. Third quarter GAAP net income was $21.6 million, compared to $26.3 million in the same period last year. Excluding non-cash expenses related to stock-based compensation and the utilization of deferred tax assets, together with related income tax effects, non-GAAP net income in the third quarter was $30.0 million, compared to $37.8 million in the third quarter of 2019. A reconciliation of GAAP to non-GAAP net income is included below. Corcept narrowed its 2020 revenue guidance range to $355 – 365 million. -

Efficacy and Safety of the Selective Glucocorticoid Receptor Modulator
Efficacy and Safety of the Selective Glucocorticoid Receptor Modulator, Relacorilant (up to 400 mg/day), in Patients With Endogenous Hypercortisolism: Results From an Open-Label Phase 2 Study Rosario Pivonello, MD, PhD1; Atil Y. Kargi, MD2; Noel Ellison, MS3; Andreas Moraitis, MD4; Massimo Terzolo, MD5 1Università Federico II di Napoli, Naples, Italy; 2University of Miami Miller School of Medicine, Miami, FL, USA; 3Trialwise, Inc, Houston, TX, USA; 4Corcept Therapeutics, Menlo Park, CA, USA; 5Internal Medicine 1 - San Luigi Gonzaga Hospital, University of Turin, Orbassano, Italy INTRODUCTION EFFICACY ANALYSES Table 5. Summary of Responder Analysis in Patients at Last SAFETY Relacorilant is a highly selective glucocorticoid receptor modulator under Key efficacy assessments were the evaluation of blood pressure in the Observation Table 7 shows frequency of TEAEs; all were categorized as ≤Grade 3 in hypertensive subgroup and glucose tolerance in the impaired glucose severity investigation for the treatment of all etiologies of endogenous Cushing Low-Dose Group High-Dose Group tolerance (IGT)/type 2 diabetes mellitus (T2DM) subgroup Five serious TEAEs were reported in 4 patients in the high-dose group syndrome (CS) Responder, n/N (%) Responder, n/N (%) o Relacorilant reduces the effects of cortisol, but unlike mifepristone, does o Response in hypertension defined as a decrease of ≥5 mmHg in either (pilonidal cyst, myopathy, polyneuropathy, myocardial infarction, and not bind to progesterone receptors (Table 1)1 mean systolic blood pressure (SBP) or diastolic blood pressure (DBP) from HTN 5/12 (41.67) 7/11 (63.64) hypertension) baseline No drug-induced cases of hypokalemia or abnormal vaginal bleeding were IGT/T2DM 2/13 (15.38) 6/12 (50.00) o Response in IGT/T2DM defined by one of the following: noted Table 1. -

A Validated Predictive Algorithm of Post-Traumatic Stress Course Following Emergency Department Admission After a Traumatic Stressor
LETTERS https://doi.org/10.1038/s41591-020-0951-z A validated predictive algorithm of post-traumatic stress course following emergency department admission after a traumatic stressor Katharina Schultebraucks 1,2,3 ✉ , Arieh Y. Shalev1, Vasiliki Michopoulos4,5, Corita R. Grudzen6, Soo-Min Shin6, Jennifer S. Stevens 4, Jessica L. Maples-Keller4, Tanja Jovanovic7, George A. Bonanno8, Barbara O. Rothbaum4, Charles R. Marmar1,9, Charles B. Nemeroff10,11, Kerry J. Ressler4,12 and Isaac R. Galatzer-Levy1,13 Annually, approximately 30 million patients are discharged Notably, PTSD comes with long-term clinical and pecuniary costs from the emergency department (ED) after a traumatic to both the individual and the healthcare system. While empirically event1. These patients are at substantial psychiatric risk, validated treatments are effective in reducing the risk for PTSD6,8,9, with approximately 10–20% developing one or more disor- early prevention strategies are typically not implemented due to the ders, including anxiety, depression or post-traumatic stress lack of established methods for timely and reliable risk identifica- disorder (PTSD)2–4. At present, no accurate method exists to tion11. The ED visit is often the sole contact of trauma survivors predict the development of PTSD symptoms upon ED admis- with the healthcare system and the time immediately after trauma sion after trauma5. Accurate risk identification at the point opens a critical window to prevent the development of PTSD11,30. of treatment by ED services is necessary to inform the tar- Accurate identification of risk for PTSD during ED evaluation using geted deployment of existing treatment6–9 to mitigate sub- algorithms running on accessible data sources would provide new sequent psychopathology in high-risk populations10,11. -

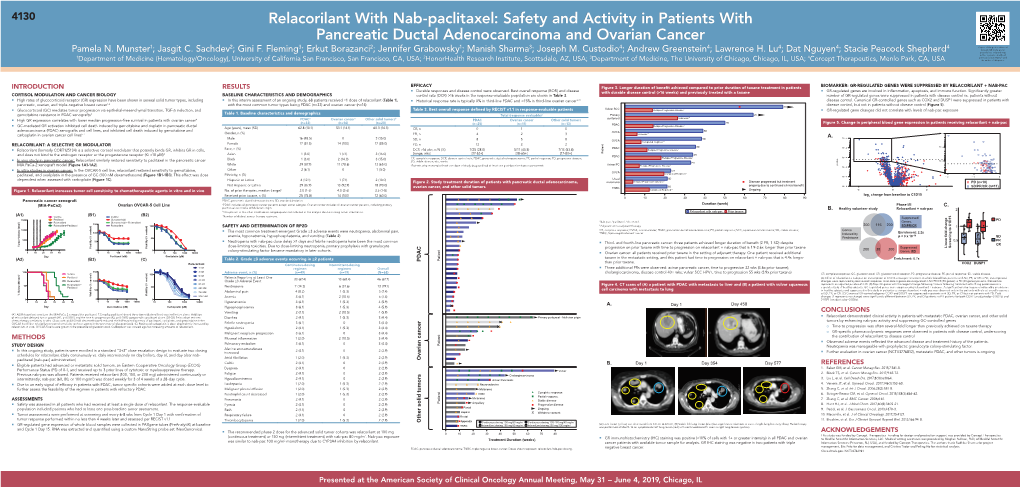
Andrew E. Greenstein1; Pamela N. Munster2; Jasgit C. Sachdev3; Gini
155 Impact of Relacorilant, a Selective Glucocorticoid Receptor Antagonist, on the Immunosuppressive Effects of Endogenous Cortisol 1 2 3 4 1 5 Copies of this poster obtained Andrew E. Greenstein ; Pamela N. Munster ; Jasgit C. Sachdev ; Gini F. Fleming ; Andreas Grauer ; Stacie Peacock Shepherd through Quick Response (QR) Code are for personal use only and may not be reproduced 1 2 3 without permission from ASCO® Corcept Therapeutics, Menlo Park, CA, USA; University of California, San Francisco School of Medicine, San Francisco, CA, USA; HonorHealth Research Institute/TGen, Scottsdale, AZ, USA; and the author of this poster. 4The University of Chicago Medicine, Chicago, IL, USA; 5formerly Corcept Therapeutics, Menlo Park, CA, USA Contact: [email protected] RESULTS RELACORILANT PROMOTES CHECKPOINT INHIBITOR RESPONSE IN TRANSCRIPTIONAL AND HEMATOLOGICAL EFFECTS OF Immune Activation and Antagonism of GC Activity by Relacorilant in a Figure 11. Plasma and Whole Blood Trends in Patients with Durable Benefit. BACKGROUND o In melanomas: SYNGENEIC MOUSE MODEL RELACORILANT SUGGEST IMPROVED IMMUNE ACTIVITY IN PHASE Patient with Complete Response GR EXPRESSION IS ASSOCIATED WITH TUMOR INFILTRATION OF The combination of a PD-1 antagonist antibody (αPD1, an ICI) and relacorilant was assessed in 1/2 STUDY IN PATIENTS WITH ADVANCED SOLID TUMORS In the Phase 1/2 solid tumor study, complete response per RECIST 1.1 was observed in CORTISOL EFFECTOR AND REGULATORY IMMUNE CELLS – Positive correlations between GR and CD8+ cytotoxic T-cells (R=0.24, Neutrophil-to-Lymphocyte P<0.001), T (R=0.29, P<0.001), and T-helper type 2 (Th2) cells (R=0.2, the EG7 tumor model. -

2014-15 Catalog
Palo Alto University Palo Palo Alto University 1791 Arastradero Road Palo Alto University Palo Alto, CA 94304-1337 Ph: (800) 818-6136 Fax: (650) 433-3888 2014-2015 Catalog 2014-2015 Catalog 2014_Cover.indd 2-3 8/13/14 10:18 PM PALO ALTO UNIVERSITY 2014-15 CATALOG 1 TABLE OF CONTENTS Graduate Fellowships (Grants) ........................................................................27 Federal Pell Grants (Undergraduate students) ................................................27 Federal Supplemental Opportunity Grant (Undergraduate students) ..............27 SECTION I – INTRODUCTION TO .............................................................13 Student Employment ........................................................................................28 Student Loans ..................................................................................................28 PALO ALTO UNIVERSITY (PAU) Satisfactory Academic Progress:......................................................................29 SAP Standards for Graduate Programs ...........................................................29 Palo Alto University Profile ......................................................................15 Cumulative Minimum Grade Point Average: .................................................29 Overview...........................................................................................................15 Average Credit Units per Quarter (Full time students only): ........................30 Core Purpose ...................................................................................................15 -

CORCEPT THERAPEUTICS INCORPORATED (Exact Name of Corporation As Specified in Its Charter)
Table of Contents As filed with the Securities and Exchange Commission on April 14, 2004 Registration No. 333-112676 SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 AMENDMENT NO. 3 to FORM S-1 REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933 CORCEPT THERAPEUTICS INCORPORATED (Exact Name of Corporation as Specified in Its Charter) Delaware 2834 77-0487658 (State or other jurisdiction of (Primary Standard Industrial (I.R.S. Employer Identification No.) incorporation or organization) Classification Code Number) Corcept Therapeutics Incorporated 275 Middlefield Road, Suite A Menlo Park, CA 94025 (650) 327-3270 (Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices) Joseph K. Belanoff, M.D. Chief Executive Officer Corcept Therapeutics Incorporated 275 Middlefield Road, Suite A Menlo Park, CA 94025 (650) 327-3270 (Name, address, including zip code, and telephone number, including area code, of agent for service) Copies to: Sarah A. O’Dowd Patrick T. Seaver Kyle Guse B. Shayne Kennedy Heller Ehrman White & McAuliffe LLP Latham & Watkins LLP 275 Middlefield Road 650 Town Center Drive Menlo Park, California 94025 Costa Mesa, California 92626 Telephone: (650) 324-7000 Telephone: (714) 540-1235 Facsimile: (650) 324-0638 Facsimile: (714) 755-8290 Approximate date of commencement of proposed sale to the public: As soon as practicable following the effectiveness of this Registration Statement. If any of the securities being registered on this Form are to be offered on a delayed -

CORCEPT THERAPEUTICS INCORPORATED (Exact Name of Corporation As Specified in Its Charter)
Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-Q x QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended June 30, 2010 or ¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number: 000-50679 CORCEPT THERAPEUTICS INCORPORATED (Exact Name of Corporation as Specified in Its Charter) Delaware 77-0487658 (State or other jurisdiction of (I.R.S. Employer Identification No.) incorporation or organization) 149 Commonwealth Drive Menlo Park, CA 94025 (Address of principal executive offices, including zip code) (650) 327-3270 (Registrant’s telephone number, including area code) Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨ Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨ Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. -

Corcept Therapeutics Announces Poster Presentations On
Corcept Therapeutics Announces Poster Presentations on Mifepristone for the Treatment of Patients with Cushing’s Syndrome at the 27th Annual American Association of Clinical Endocrinologists May 14, 2015 Corcept Therapeutics Incorporated (NASDAQ: CORT), a pharmaceutical company engaged in the discovery, development and commercialization of drugs for the treatment of severe metabolic, oncologic and psychiatric disorders, today announced that four posters about mifepristone will be presented at the 24th annual American Association of Clinical Endocrinologists (AACE) being held at the Music City Convention Center in Nashville, TN from May 13 – 17, 2015. “We are pleased that physicians have taken a strong interest in mifepristone and its potential to treat patients with Cushing’s syndrome,” said Joseph K. Belanoff, M.D., Corcept’s Chief Executive Officer. In addition to presentation of the four abstracts described below, AACE attendees will have the opportunity to attend a “Product Theatre” talk by Ty Carroll, M.D., whose topic will be “Not-So-Subclinical Cushing’s Syndrome.” Corcept is a sponsor of Dr. Carroll’s presentation. Title Authors Friday, May 15, 2015 9:45am – 2:15pm Poster #131: “Resistant metabolic abnormalities prompting Cushing’s syndrome Douglas Beatty, M.D., Rachel work-up and treatment with mifepristone” Bunta, Dat Nguyen, PharmD Poster #840: “Mifepristone re-established glycemic control in a Cushing’s syndrome Todd Frieze, M.D., James Smith, patient that relapsed after a 21 month interruption” Ph. D. Friday, May 15, 2015 10:00am – 10:45am Product Theater A Product Theater: “Not-So-Subclinical Cushing’s Syndrome” Ty Carroll, M.D. Saturday, May 16, 2015 9:45am – 1:00pm Late-Breaking Poster: “Use of mifepristone in an ectopic Cushing’s syndrome patient Adeela Ansari, M.D., Precious Lim, pending tumor localization” Ph.D., Dat Nguyen, PharmD Late-Breaking Poster: “Improved response to octreotide LAR for ectopic Cushing’s Andreas Moraitis, M.D., Richard syndrome during mifepristone therapy: A case study” Auchus, M.D. -

CORCEPT THERAPEUTICS INCORPORATED (Exact Name of Corporation As Specified in Its Charter)
Table of Contents As filed with the Securities and Exchange Commission on March 19, 2004 Registration No. 333-112676 SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 AMENDMENT NO. 1 to FORM S-1 REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933 CORCEPT THERAPEUTICS INCORPORATED (Exact Name of Corporation as Specified in Its Charter) Delaware 2834 77-0487658 (State or other jurisdiction of (Primary Standard Industrial (I.R.S. Employer Identification No.) incorporation or organization) Classification Code Number) Corcept Therapeutics Incorporated 275 Middlefield Road, Suite A Menlo Park, CA 94025 (650) 327-3270 (Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices) Joseph K. Belanoff, M.D. Chief Executive Officer Corcept Therapeutics Incorporated 275 Middlefield Road, Suite A Menlo Park, CA 94025 (650) 327-3270 (Name, address, including zip code, and telephone number, including area code, of agent for service) Copies to: Sarah A. O’Dowd Patrick T. Seaver Kyle Guse B. Shayne Kennedy Heller Ehrman White & McAuliffe LLP Latham & Watkins LLP 275 Middlefield Road 650 Town Center Drive Menlo Park, California 94025 Costa Mesa, California 92626 Telephone: (650) 324-7000 Telephone: (714) 540-1235 Facsimile: (650) 324-0638 Facsimile: (714) 755-8290 Approximate date of commencement of proposed sale to the public: As soon as practicable following the effectiveness of this Registration Statement. If any of the securities being registered on this Form are to be offered on a delayed