082 Genus Tarsocera Butler
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title Lorem Ipsum Dolor Sit Amet, Consectetur Adipiscing Elit
Volume 26: 102–108 METAMORPHOSIS www.metamorphosis.org.za ISSN 1018–6490 (PRINT) LEPIDOPTERISTS’ SOCIETY OF AFRICA ISSN 2307–5031 (ONLINE) Classification of the Afrotropical butterflies to generic level Published online: 25 December 2015 Mark C. Williams 183 van der Merwe Street, Rietondale, Pretoria, South Africa. E-mail: [email protected] Copyright © Lepidopterists’ Society of Africa Abstract: This paper applies the findings of phylogenetic studies on butterflies (Papilionoidea) in order to present an up to date classification of the Afrotropical butterflies to genus level. The classification for Afrotropical butterflies is placed within a worldwide context to subtribal level. Taxa that still require interrogation are highlighted. Hopefully this classification will provide a stable context for researchers working on Afrotropical butterflies. Key words: Lepidoptera, Papilionoidea, Afrotropical butterflies, classification. Citation: Williams, M.C. (2015). Classification of the Afrotropical butterflies to generic level. Metamorphosis 26: 102–108. INTRODUCTION Suborder Glossata Fabricius, 1775 (6 infraorders) Infraorder Heteroneura Tillyard, 1918 (34 Natural classifications of biological organisms, based superfamilies) on robust phylogenetic hypotheses, are needed before Clade Obtectomera Minet, 1986 (12 superfamilies) meaningful studies can be conducted in regard to their Superfamily Papilionoidea Latreille, 1802 (7 evolution, biogeography, ecology and conservation. families) Classifications, dating from the time of Linnaeus in the Family Papilionidae Latreille, 1802 (32 genera, 570 mid seventeen hundreds, were based on morphology species) for nearly two hundred and fifty years. Classifications Family Hedylidae Guenée, 1858 (1 genus, 36 species) based on phylogenies derived from an interrogation of Family Hesperiidae Latreille, 1809 (570 genera, 4113 the genome of individual organisms began in the late species) 20th century. -

Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier Area, Swellendam
Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier area, Swellendam by Johannes Philippus Groenewald Thesis presented in fulfilment of the requirements for the degree of Masters in Science in Conservation Ecology in the Faculty of AgriSciences at Stellenbosch University Supervisor: Prof. Michael J. Samways Co-supervisor: Dr. Ruan Veldtman December 2014 Stellenbosch University http://scholar.sun.ac.za Declaration I hereby declare that the work contained in this thesis, for the degree of Master of Science in Conservation Ecology, is my own work that have not been previously published in full or in part at any other University. All work that are not my own, are acknowledge in the thesis. ___________________ Date: ____________ Groenewald J.P. Copyright © 2014 Stellenbosch University All rights reserved ii Stellenbosch University http://scholar.sun.ac.za Acknowledgements Firstly I want to thank my supervisor Prof. M. J. Samways for his guidance and patience through the years and my co-supervisor Dr. R. Veldtman for his help the past few years. This project would not have been possible without the help of Prof. H. Geertsema, who helped me with the identification of the Lepidoptera and other insect caught in the study area. Also want to thank Dr. K. Oberlander for the help with the identification of the Oxalis species found in the study area and Flora Cameron from CREW with the identification of some of the special plants growing in the area. I further express my gratitude to Dr. Odette Curtis from the Overberg Renosterveld Project, who helped with the identification of the rare species found in the study area as well as information about grazing and burning of Renosterveld. -

Report on the Terrestrial Ecology (Flora and Fauna)
Kudusberg WEF REPORT ON THE TERRESTRIAL ECOLOGY (FLORA AND FAUNA) Basic Assessment report for the proposed development of the 325 MW Kudusberg Wind Energy Facility located west of the R354 Between Matjiesfontein and Sutherland in the Northern and Western Cape Report prepared for: Report prepared by: CSIR – Environmental Management Services Ekotrust cc (CK90/05465/23) P O Box 17001 7 St George Street, Lionviham Congella, Durban, 4013 Somerset West 7130 South Africa South Africa OCTOBER 2018 Ekotrust CC - 2018 1 CONTENTS EXECUTIVE SUMMARY .................................................................................................................... i SPECIALISTS DECLARATION ........................................................................................................... iv TERMS OF REFERENCE ................................................................................................................... v LIMITATIONS, ASSUMPTIONS & UNCERTAINTIES ........................................................................ vi ACRONYMS ................................................................................................................................... vii ABBREVIATIONS ........................................................................................................................... vii GLOSSARY .................................................................................................................................... viii LIST OF FIGURES ........................................................................................................................... -

2 Title 3 the Trials and Tribulations of Priors and Posteriors in B
bioRxiv preprint doi: https://doi.org/10.1101/259184; this version posted February 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Running head 2 THE AGE OF BUTTERFLIES REVISITED (AND TESTED) 3 Title 4 The Trials and Tribulations of Priors and Posteriors in Bayesian Timing of 5 Divergence Analyses: the Age of Butterflies Revisited. 6 7 Authors 8 NICOLAS CHAZOT1*, NIKLAS WAHLBERG1, ANDRÉ VICTOR LUCCI FREITAS2, 9 CHARLES MITTER3, CONRAD LABANDEIRA3,4, JAE-CHEON SOHN5, RANJIT KUMAR 10 SAHOO6, NOEMY SERAPHIM7, RIENK DE JONG8, MARIA HEIKKILÄ9 11 Affiliations 12 1Department of Biology, Lunds Universitet, Sölvegatan 37, 223 62, Lund, Sweden. 13 2Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de 14 Campinas (UNICAMP), Cidade Universitária Zeferino Vaz, Caixa postal 6109, 15 Barão Geraldo 13083-970, Campinas, SP, Brazil. 16 3Department of Entomology, University of Maryland, College Park, MD 20742, U.S.A. 17 4Department of Paleobiology, National Museum of Natural History, Smithsonian 18 Institution, Washington, DC 20013, USA; Department of Entomology and BEES 19 Program, University of Maryland, College Park, MD 20741; and Key Lab of Insect 20 Evolution and Environmental Change, School of Life Sciences, Capital Normal 21 University, Beijing 100048, bioRxiv preprint doi: https://doi.org/10.1101/259184; this version posted February 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Running Head 1 the AGE of BUTTERFLIES REVISITED
bioRxiv preprint doi: https://doi.org/10.1101/259184; this version posted February 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Running head 2 THE AGE OF BUTTERFLIES REVISITED (AND TESTED) 3 Title 4 The Trials and Tribulations of Priors and Posteriors in Bayesian Timing of 5 Divergence Analyses: the Age of Butterflies Revisited. 6 7 Authors 8 NICOLAS CHAZOT1*, NIKLAS WAHLBERG1, ANDRÉ VICTOR LUCCI FREITAS2, 9 CHARLES MITTER3, CONRAD LABANDEIRA3,4, JAE-CHEON SOHN5, RANJIT KUMAR 10 SAHOO6, NOEMY SERAPHIM7, RIENK DE JONG8, MARIA HEIKKILÄ9 11 Affiliations 12 1Department of Biology, Lunds Universitet, Sölvegatan 37, 223 62, Lund, Sweden. 13 2Departamento de Biologia Animal, Instituto de Biologia, Universidade Estadual de 14 Campinas (UNICAMP), Cidade Universitária Zeferino Vaz, Caixa postal 6109, 15 Barão Geraldo 13083-970, Campinas, SP, Brazil. 16 3Department of Entomology, University of Maryland, College Park, MD 20742, U.S.A. 17 4Department of Paleobiology, National Museum of Natural History, Smithsonian 18 Institution, Washington, DC 20013, USA; Department of Entomology and BEES 19 Program, University of Maryland, College Park, MD 20741; and Key Lab of Insect 20 Evolution and Environmental Change, School of Life Sciences, Capital Normal 21 University, Beijing 100048, bioRxiv preprint doi: https://doi.org/10.1101/259184; this version posted February 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Download Document
SANBI Biodiversity Series 16 Butterflies of South Africa’s National Botanical Gardens An illustrated checklist compiled by Christopher K. Willis & Steve E. Woodhall Pretoria 2010 SANBI Biodiversity Series The South African National Biodiversity Institute (SANBI) was established on 1 Sep- tember 2004 through the signing into force of the National Environmental Manage- ment: Biodiversity Act (NEMBA) No. 10 of 2004 by President Thabo Mbeki. The Act expands the mandate of the former National Botanical Institute to include responsibili- ties relating to the full diversity of South Africa’s fauna and flora, and builds on the internationally respected programmes in conservation, research, education and visitor services developed by the National Botanical Institute and its predecessors over the past century. The vision of SANBI: Biodiversity richness for all South Africans. SANBI’s mission is to champion the exploration, conservation, sustainable use, appre- ciation and enjoyment of South Africa’s exceptionally rich biodiversity for all people. SANBI Biodiversity Series publishes occasional reports on projects, technologies, work- shops, symposia and other activities initiated by or executed in partnership with SANBI. Photographs: Steve Woodhall, unless otherwise noted Technical editing: Emsie du Plessis Design & layout: Sandra Turck Cover design: Sandra Turck Cover photographs: Front: Pirate (Christopher Willis) Back, top: African Leaf Commodore (Christopher Willis) Back, centre: Dotted Blue (Steve Woodhall) Back, bottom: Green-veined Charaxes (Christopher Willis) Citing this publication WILLIS, C.K. & WOODHALL, S.E. (Compilers) 2010. Butterflies of South Africa’s National Botanical Gardens. SANBI Biodiversity Series 16. South African National Biodiversity Institute, Pretoria. ISBN 978-1-919976-57-0 © Published by: South African National Biodiversity Institute. -

395 Genus Tarsocera Butler
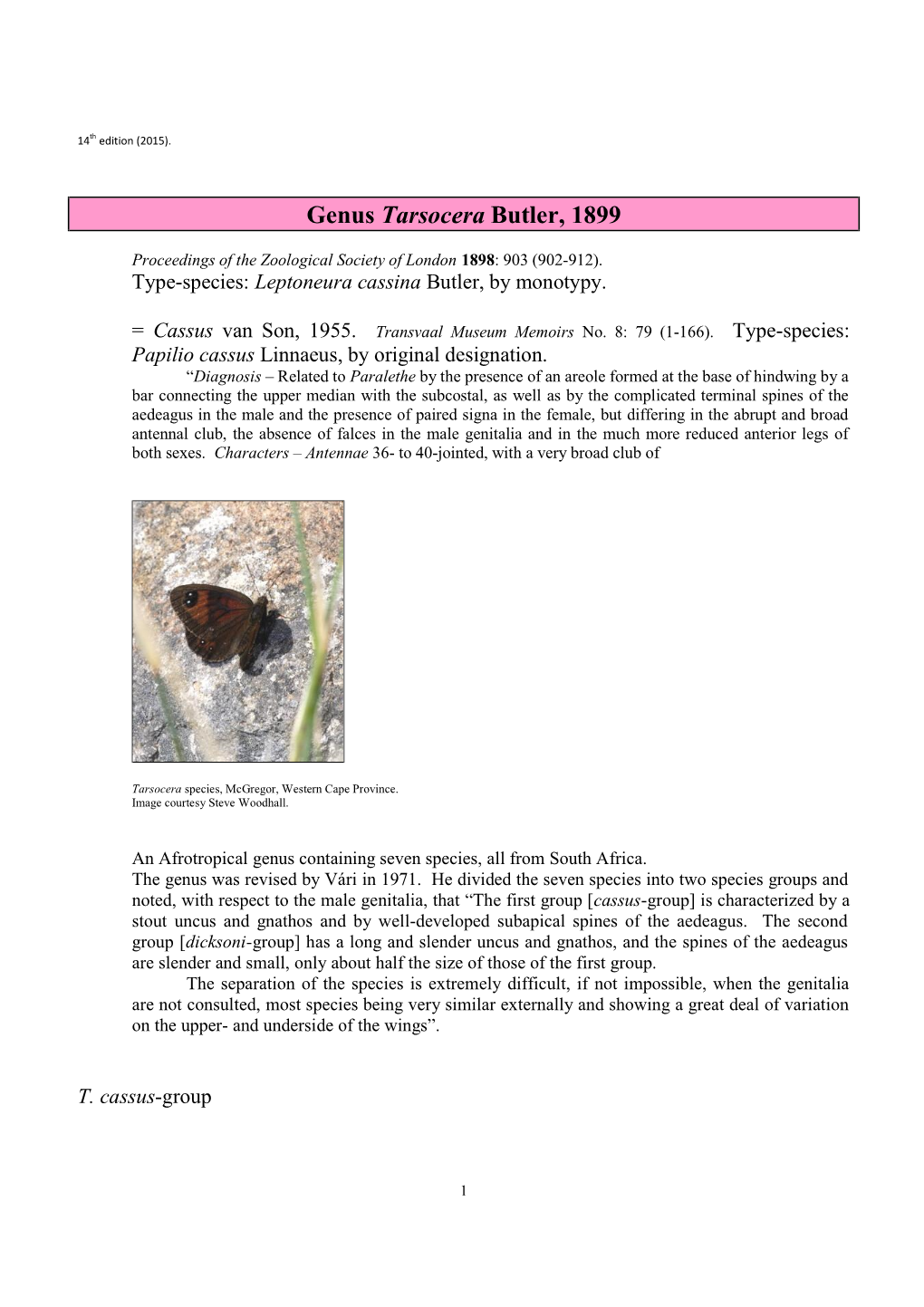
AFROTROPICAL BUTTERFLIES. MARK C. WILLIAMS. http://www.lepsocafrica.org/?p=publications&s=atb Updated 18 January 2019 Genus Tarsocera Butler, 1899 Spring Widows Proceedings of the Zoological Society of London 1898: 903 (902-912). Type-species: Leptoneura cassina Butler, by monotypy. = Cassus van Son, 1955. Transvaal Museum Memoirs No. 8: 79 (1-166). Type-species: Papilio cassus Linnaeus, by original designation. “Diagnosis – Related to Paralethe by the presence of an areole formed at the base of hindwing by a bar connecting the upper median with the subcostal, as well as by the complicated terminal spines of the aedeagus in the male and the presence of paired signa in the female, but differing in the abrupt and broad antennal club, the absence of falces in the male genitalia and in the much more reduced anterior legs of both sexes. Characters – Antennae 36- to 40-jointed, with a very broad club of Tarsocera species, McGregor, Western Cape Province. Image courtesy Steve Woodhall. The genus Tarsocera belongs to the Family Nymphalidae Rafinesque, 1815; Subfamily Satyrinae Boisduval, 1833; Tribe Dirini Verity, 1953. The other genera in the Tribe Dirini in the Afrotropical Region are Paralethe, Aeropetes, Torynesis, Dira, Serradinga and Dingana. Tarsocera (Spring Widows) is an Afrotropical genus containing seven species, all from South Africa. The genus was revised by Vári in 1971. He divided the seven species into two species groups and noted, with respect to the male genitalia, that “The first group [cassus-group] is characterized by a stout uncus and gnathos and by well-developed subapical spines of the aedeagus. The second group [dicksoni-group] has a long and slender uncus and gnathos, and the spines of the aedeagus are slender and small, only about half the size of those of the first group. -

132 Genus Torynesis Butler
AFROTROPICAL BUTTERFLIES 17th edition (2018). MARK C. WILLIAMS. http://www.lepsocafrica.org/?p=publications&s=atb Genus Torynesis Butler, 1899 Proceedings of the Zoological Society of London 1898: 903 (902-912). Type-species: Dira mintha Geyer, by monotypy. = Mintha van Son, 1955. Transvaal Museum Memoirs No. 8: 76 (1-166). Type-species: Dira mintha Geyer, by original designation. The genus Torynesis belongs to the Family Nymphalidae Rafinesque, 1815; Subfamily Satyrinae Boisduval, 1833; Tribe Dirini Verity, 1953. The other genera in the Tribe Dirini in the Afrotropical Region are Paralethe, Aeropetes, Tarsocera, Dira, Serradinga and Dingana. Torynesis (Widows) is an Afrotropical genus containing five species, from South Africa and Lesotho. Torynesis species identification plate Torynesis hawequas male Torynesis hawequas female Torynesis mintha mintha male Toryne sis mintha mintha female Torynesis mintha piquetbergensis male Torynesis mintha piquetbergensis female 1 Torynesis magna male Toryne sis magna female Torynesis pringlei male Torynes is pringlei female Torynesis orangica male Toryne sis orangica female *Torynesis hawequas Dickson, 1973# Hawequas Widow Hawequas Widow (Torynesis hawequas) female, Franschoek Pass, Western Cape Province. Image courtesy Steve Woodhall. Torynesis hawequas Dickson, 1973. Entomologist’s Record and Journal of Variation 85: 284 (284-288). Torynesis hawequas Dickson, 1973. Dickson & Kroon, 1978. Torynesis hawequas Dickson, 1973. Pringle et al., 1994: 57. 2 Torynesis hawequas. Male (Wingspan 47 mm). Left – upperside; right – underside. Franschhoek Pass, Western Cape Province, South Africa. 20 April 1975. I. Bampton. Images M.C.Williams ex Henning Collection. Torynesis hawequas. Female (Wingspan 51 mm). Left – upperside; right – underside. Franschhoek Mountain, Western Cape Province, South Africa. 20 April 1975. I. -
Download Download
Biodiversity Observations http://bo.adu.org.za An electronic journal published by the Animal Demography Unit at the University of Cape Town The scope of Biodiversity Observations consists of papers describing observations about biodiversity in general, including animals, plants, algae and fungi. This includes observations of behaviour, breeding and flowering patterns, distributions and range extensions, foraging, food, movement, measurements, habitat and colouration/plumage variations. Biotic interactions such as pollination, fruit dispersal, herbivory and predation fall within the scope, as well as the use of indigenous and exotic species by humans. Observations of naturalised plants and animals will also be considered. Biodiversity Observations will also publish a variety of other interesting or relevant biodiversity material: reports of projects and conferences, annotated checklists for a site or region, specialist bibliographies, book reviews and any other appropriate material. Further details and guidelines to authors are on this website. Lead Editor: Arnold van der Westhuizen – Paper Editor: Les G Underhill REPORT ON THE SPRING LEPIBASH FOR LEPIMAP, 15–23 OCTOBER 2016 Megan Loftie-Eaton Recommended citation format: Loftie-Eaton M 2016 Report on the Spring LepiBASH for LepiMAP, 15–23 October 2016. Biodiversity Observations 7.89: 1–11. URL: http://bo.adu.org.za/content.php?id=282 Published online: 11 December 2016 – ISSN 2219-0341 – Biodiversity Observations 7.89: 1–11 1 PROJECT REPORT http://internal.adu.org.za/upload/uploads/Megan-Loftie-Eaton-and- Lepibash_2016-10-22.mp3. REPORT ON THE SPRING LEPIBASH FOR LEPIMAP, RESULTS 15–23 OCTOBER 2016 Megan Loftie-Eaton Numerical Animal Demography Unit, Department of Biological Sciences, During the nine days of the Spring LepiBASH, a total of 85 observers University of Cape Town, Rondebosch, 7701 South Africa participated and a total of 2080 records were submitted. -

Evolution of Host Repertoires and the Diversification of Butterflies
Evolution of host repertoires and the diversification of butterflies Mariana Pires Braga Academic dissertation for the Degree of Doctor of Philosophy in Animal Ecology at Stockholm University to be publicly defended on Friday 15 February 2019 at 10.00 in Vivi Täckholmsalen (Q-salen), NPQ-huset, Svante Arrhenius väg 20. Abstract All herbivorous insects are specialized to some extent to their host plants, but the level of specialization varies greatly. Insect-plant coevolution is often invoked to explain the large diversity of herbivorous insects, but the role of specialization during diversification is still controversial. Although well-studied, our understanding of the evolution of species interactions is still improving, and recent theoretical developments have highlighted the role of generalization (via colonization of new hosts) on diversification. In this thesis, various approaches are combined for a detailed study of the origins of macroevolutionary patterns of host use and butterfly diversity. Chapter I provides a mechanistic basis for such patterns through simulations of lineages evolved in silico. By separating the effects of the number of hosts used by a parasite lineage and the diversity of resources they encompass, we found that resource diversity, rather than host range per se, was the main driver of parasite species richness in both simulated and empirical systems. In Chapter II, we combined network and phylogenetic analyses to quantify support for the two main hypothesized drivers of diversification of herbivorous insects. Based on analyses of two butterfly families, Nymphalidae and Pieridae, we found that variability in host use is essential for diversification, while radiation following the colonization of a new host is rare but can produce high diversity. -

004 Afrotropical Butterfly Classification
AFROTROPICAL BUTTERFLIES. MARK C. WILLIAMS. http://www.lepsocafrica.org/?p=publications&s=atb Updated 21 August 2021 CLASSIFICATION OF THE AFROTROPICAL BUTTERFLIES TO GENERIC LEVEL Updated from Williams, 2015 (Metamorphosis 26: 102-108). Taxa highlighted in yellow have been added since Williams, 2015. Taxa highlighted in green have been removed since Williams, 2015. The Superfamily PAPILIONOIDEA (Butterflies) has a total of about 19 000 species in about 1 820 genera. In the Afrotropical Region there are 4 515 species (21 August 2021) (about 23% of the world total) in 337 genera (about 18% of the world total). FAMILY PAPILIONIDAE Latreille, [1802]. [103 species in 3 genera] This is the second smallest family (after the Hedylidae) in the Papilionoidea. The family is cosmopolitan, with about 570 species in 32 genera. There are 101 species in 3 genera in the Afrotropical Region. Subfamily Papilioninae Latreille, [1802]. [103 species] Tribe Leptocircini Kirby, 1896. [42 species] Afrotropical genus: Graphium (42 species) Tribe Papilionini Latreille, [1802]. [60 species] Afrotropical genus: Papilio (60 species) Tribe Troidini Talbot, 1939. [1 species] Subtribe Troidina Talbot, 1939. [1 species] Afrotropical genus: Pharmacophagus (1 species) FAMILY HESPERIIDAE Latreille, 1809. [625 species in 95 genera] This family is cosmopolitan, with about 4, 150 species in 570 genera. There are 625 species in 94 genera in the Afrotropical Region. Subfamily Coeliadinae Evans, 1937. [21 species in 3 genera] Afrotropical genera: Coeliades (19 species), Tekliades (1 species), Pyrrhiades, Pyrrhochalcia (1 species) Subfamily Katreinae Grishin, 2019. [5 species in 2 genera] Afrotropical genera: Ortholexis (4 species), Katreus (1 species). Subfamily Tagiadinae Mabille, 1878. [191 species in 17 genera] Tribe Celaenorrhinini Swinhoe, 1912. -

DEIR APP E14 Invertibrate Faunal Assessment
ENVIRONMENTAL IMPACT ASSESSMENT FOR THE PROPOSED NUCLEAR POWER STATION (‘NUCLEAR 1’) AND ASSOCIATED INFRASTRUCTURE Terrestrial Invertebrate Fauna Assessment November 2010 Prepared by: Afribugs c.c. Prepared for: Arcus GIBB Pty Ltd On behalf of: Eskom Holdings Ltd Reg. No. 1999/032723/23 VAT Reg. No 4840234696 P.G. Hawkes B.Sc. (Hons) Pr.Sci.Nat 379 Bakenkloof Street Wolmer, Pretoria North 0182, South Africa Tel: +27 12 546 7289 Mobile: +27 72 133 8677 Fax: +27 86 670 9055 email: [email protected] Website: www.afribugs.com Specialist invertebrate studies EIA / biodiversity surveys Rehabilitation monitoring 18 April 2011 DECLARATION OF INDEPENDENCE I, _Peter Geoffrey Hawkes, as duly authorised representative of Afribugs, hereby confirm my independence (as well as that of Afribugs) as a specialist and declare that neither I nor Afribugs have any interest, be it business, financial, personal or other, in any proposed activity, application or appeal in respect of which Arcus GIBB was appointed as environmental assessment practitioner in terms of the National Environmental Management Act, 1998 (Act No. 107 of 1998), other than fair remuneration for worked performed, specifically in connection with the Environmental Impact Assessment for the proposed conventional nuclear power station (‘Nuclear 1’). I further declare that I am confident in the results of the studies undertaken and conclusions drawn as a result of it, subject only to declared limitations – as is described in my attached report. ______________________________ Full Name: