Paper Template
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Sl No App.No 1 5291 2 5292 3 5293 4 5294 5 5295 6 5296 7 5297 8 5298
Page 1 of 67 SL APP.NO CANDIDATE NAME NO AND ADDRESS MANIKANDANATH N, S/O NADARAJAN,, PONNARAI, 1 5291 SAHAYA NAGAR, PALAPALLAM (VIA), KANYAKUMARI- 629159 SATHEESH KUMAR K.R, S/O.KUMARADHA S,, 2 5292 VARAGU VILAI,, BETHELPURAM POST,, KANYAKUMARI-0 SHIJU R, S/O.RAMALINGAM NADAR, 3 5293 KAVU VILAI HOUSE,, METHUKUMMAL POST,, S.T.MANKAD, KANYAKUMARI- 629172 ROBINSON R, S/O ROBINSON[L], 4 5294 4-139, APPATTU VILAI, KAPPUKAD POST, KANYAKUMARI- 629162 SELVAKUMAR.T, S/O S.THANGAPPAN, 5 5295 NORTH STREET, MYLAUDY POST, KANYAKUMARI- 629403 NESA RAJA KUMAR, S/O.NESAMON I, 6 5296 KUZHIVILAI HOUSE, THENGAPATTANAM POST, KANYAKUMARI- 0 JAGADEESAN A, S/O AYYAPPA [LATE], 7 5297 1/120B, AKSHARA BAVAN, KRISHNAMANGALAM, THUCKALAY KANYAKUMARI- 629175 MANOHARAN A, S/O ARIKRISHNA PERUMAL, 8 5298 D.NO.3-4,, ATHIKATTU VILAI, MONIKETTIPOTTAL POST- KANYAKUMARI- 629501 Page 2 of 67 NELSON A, S/O ALLECY, 9 5299 KONATHU VILAI, KOODAITHUCKY ROAD, KULASEKHARAM POST KANYAKUMARI- 629161 JOHN BENNET.N, S/O NESAMANI .N 10 5300 MAN PATTAN VILAI, CHERUKOLE, KATTATHURAI POST, KANYAKUMARI- 629158 DAVINSON.C.R, DAVIS COTTAGE, 11 5301 KUTHIRAI VAIKALI VEEDU KOLLAL, KANJAMPURAM POST KANYAKUMARI- 629154 JAYAKUMAR.N, S/O S.NARAYANAN, 12 5302 4-114,PADAR NILAM, VAYAL KARAI, MANAVALAKURICHY POST KANYAKUMARI- 629252 SUNIL T, S/O THANKIAN N 13 5303 KARUMPILA VILAI HOUSE ADAIKKA KUZHI POST KALIYAKKAVILAI VIA KANYAKUMARI- 629153 SASI KUMAR P, S/O PACHAN,, 14 5304 KANCHIRA VILAGAM HOUSE, AYINKAMAMDESOM, KALIAKKAVILAI PO, KANYAKUMARI- 629153 THIYAGARAJAN.T, S/O M.THIRULINGAM, 15 5305 17- 10,CHOTHA VILAI, PUTHALAM POST, KANYAKUMARI- 629602 SREE KUMAR M, S/O.MURUGAN,, 16 5306 POOCHIKATTU VILAI,, THICKANAMCODE POST, KANYAKUMARI-0 Page 3 of 67 MANIGANDAN S, S/O SIVAGURUNATHAN,, 17 5307 19-61B PUVIYOOR,, SOUTH THAMARAIKULAM, AGASTEESWARAM POST. -

Polling Station with Voters English
List of Polling Stations For 232 Padmanabhapuram Assembly Segments within the 39 KANNIAYA KUMARI Parliamentary Constituency Sl.No Polling station No. Location and name of building in which Polling Station Polling Areas Whether located for All Voters or Men only or Women only 1 2 3 4 5 1 1 Manmakil Mantram, Kothaiyar Melthangal ,Main 1.Surulacode ( R.V ), Peachiparai ( P ) All Voters Building, EB Department Ward 1, Block 4, Kothaiyar Melthangal 2 2 Govt. M.S.Kodayar Lower Camp ,New Building, East 1.Surulacode ( R.V ), Peachiparai( P ) All Voters Portion Ward 1, Block 2,3 ,Kothaiyar Kezhelthangal , 2.Surulacode ( R.V ), Peachiparai ( P ) Ward 1, Block 4,5, Kothaiyar Kezhelthangal south 3 3 P. W.D.Building, Balamore ,Main Building, Middle 1.Surulacode ( R.V ), Balamore ( P ) Ward All Voters Portion 1, Block 1,4, Balamore , 2.Surulacode ( R.V ), Balamore ( P ) Block 4, Sambakkal 4 4 Karimoni Estate Karimoni ,Estate Old Hospital, Western 1.Surulacode ( R.V ) , Balamore ( P ) Ward All Voters Portion 1, Block 2, Karimoni , 2.Surulacode ( R.V ), Balamore ( P ) Block 3, Kaarimoni Melpakuthi 5 5 Govt. Tribal Residence Hr.Sec.School, Pechipparai ,Main 1.Surulacode ( r.v ), peachiparai ( p ) Block All Voters Building, Northern Portion 2, 5, 6, Vizhamalai 6 6 Govt. Tribal Residence Hr.Sec.School, Pechipparai ,Main 1.Surulacode ( R.V ), Peachiparai ( P ) All Voters Building, Northern side Portion Block 7, 8, 13, Peachiparai Hills List of Polling Stations For 232 Padmanabhapuram Assembly Segments within the 39 KANNIAYA KUMARI Parliamentary Constituency 7 7 Govt. Tribal Residence H.S.S.Pechipparai ,Main 1.Tiruparapu ( R.V ), Peachiparai ( P ) All Voters Building, Northern Portion Ward 1, Block 8,10, Maniyankozhi , 2.Tiruparapu ( R.V ), Peachiparai ( P ) Ward 5, Block 9,12,13, Khakatchal,Thurainallur 8 8 Govt. -
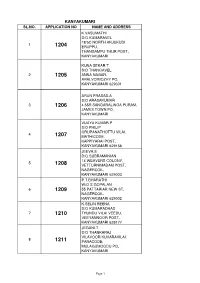
Kanyakumari Sl.No
KANYAKUMARI SL.NO. APPLICATION NO NAME AND ADDRESS K.VASUMATHI D/O KUMARAVEL 18/5C,NORTH ANJUKUDI 1 1204 ERUPPU, THANGAMPU THUR POST, KANYAKUMARI KUNA SEKAR.T S/O THANGAVEL 2 1205 ANNA NAGAR, ARALVOIMOZHY PO, KANYAKUMARI 629301 ARUN PRASAD.A S/O ARASAKUMAR 3 1206 4.55R SANGARALINGA PURAM, JAMES TOWN PO, KANYAKUMARI VIJAYA KUMAR.P S/O PHILIP ORUPANATHOTTU VILAI, 4 1207 MATHICODE, KAPPIYARAI POST, KANYAKUMARI 629156 JEEVA.S D/O SUBRAMANIAN 14 WEAVERS COLONY, 5 1208 VETTURNIMADAM POST, NAGERCOIL, KANYAKUMARI 629003 P.T.BHARATHI W/O S.GOPALAN 6 1209 55 PATTARIAR NEW ST, NAGERCOIL, KANYAKUMARI 629002 K.SELIN REENA D/O KUMARADHAS 7 1210 THUNDU VILAI VEEDU, VEEYANNOOR POST, KANYAKUMARI 629177 JEGANI.T D/O THANKARAJ VILAVOOR KUVARAVILAI, 8 1211 PARACODE, MULAGUMOODU PO, KANYAKUMARI Page 1 FELSY FREEDA.L D/O LAZER 9 1212 MALAANTHATTU VILAI, PALLIYADI POST, KANYAKUMARI 629169 CHRISTAL KAVITHA. R D/O RAJAN 10 1213 VALIYAVILAGAM HOUSE ST, MANKADU POST, KANYAKUMARI 629172 PRABHA.P D/O PADMANABHAN 11 1214 EATHENKADU, FRIDAYMARKET POST, KANYAKUMARI 629802 KALAI SELVI.N D/O NARAYANA PERUMAL 12 1215 THERIVILAI SWAMITHOPPU POST, KANYAKUMARI 629704 NAGALAKSHMI.M D/O MURUGESAN LEKSHMI BHAVAN, 13 1216 CHAKKIYANCODE, NEYYOOR POST, KANYAKUMARI 629802 BEULA.S D/O SATHIADHAS 14 1217 ANAN VILAI, KEEZHKULAM PO, KANYAKUMARI 629193 MAHESWARI.S D/O SIVACHANDRESWARAN 15 1218 1/20BTHEKKURICHI, RAJAKKAMANGALAM POST, KANYAKUMARI 629503 PREMALATHA.S W/O MURALIRAJ V.L, 460F-1, M.S.ROAD, 16 1219 SINGARATHOPPUPAR, VATHIPURAM, NAGERCOIL, KANYAKUMARI 629003 SUBASH.T S/O THANKAPPAN MANALI KATTU VILAI, 17 1220 PUTHEN VEEDU THICKA, NAMCODE PO, KANYAKUMARI 629804 Page 2 J. -

Historical Enquiry Dr.Praveen.O.K Thiruvithancode Sreeneelakantaswamy Temple (Sree Mahadevar)- Historical Enquiry
Thiruvithancode Sreeneelakantaswamy Temple (Sree Mahadevar)- Historical Enquiry Dr.Praveen.O.K Thiruvithancode Sreeneelakantaswamy Temple (Sree Mahadevar)- Historical Enquiry Dr. Praveen. O. K Asstt. Prof., Deptt. of History, Sree Kerala Varma College, Thrissur, Kerala, India. Absctract Kanyakumari District is denoted with the ancient temples. Reference to this paper The temple is either for Lord siva or Lord Vishnu with different names should be made as and incarnations. In each village there is a temple. They constructed the temples for their worship and for the worship of follows: public.Thiruvithancode are located at distance of 54km from Trivandrum. This area once commanded historical and art of Dr. Praveen. O. K generalship. The temple dedicated to Sree Mahadevar situated here bestows great honour on it the status of a pilgrim centre. The past glory Thiruvithancode of this temple still continues to hold it as one of the twelve sivalayams of Sreeneelakantaswamy erstwhile south Travancore where heavy crown of devotees in during Temple sivaratri in the month of February/March. Like the majority of its (Sree Mahadevar)- counterparts in and around the area, this temple claims an existence of many thousands of years. Its puranic foundation helps to substantiate Historical Enquiry, this fact. Keywords: Devaswom, Thiruvithancode,temple,sivalay,puranic,Srikovil, Journal Global Values, Pooja, Travancore Vol. IX, No.1, Article No. 12, pp.88-94 http://anubooks.com/ ?page_id=285 88 Journal Global Values, Vol. IX, No. 1, 2018, ISSN: (P) 0976-9447, (e) 2454-8391, Impact Factor 3.8741 (ICRJIFR) UGC Approved Journal No. 63651 Introduction The place name Thiruvithamcode has its own significance. -

Kanyakumari District Statistical Handbook – 2016
Kanyakumari District Statistical Handbook – 2016 Preface Salient Features District Profile 1. Area &Population 2. Climate & Rainfall 3. Agriculture 4. Irrigation 5. Animal Husbandary 6. Banking & Insurance 7. Co-Operative Societies 8. Civil Supplies 9. Communications 10. Electricity 11. Education 12. Fisheries 13. Handloom 14. Handicrafts 15. Health & Family Welfare 16. Housing 17. Industries 18. Factories 19. Local Bodies 20. Labour & Employment 21. Legal services 22. Libraries 23. Mining & Quarrying 24. Manufacturing 27. Non-Conventional 25. Medical Services 26. Motor Vehicles Energy 28. Police & Prison 29. Public Health 30. Printing & Publications 31. Prices Indices 32. Quality Control 33. Registration 36. Recreation & Cultural 34. Repair & Services 35. Restaurants & Hotels Services 39. Scientific Research 37. Social Welfare 38. Sanitary Services Services 40. Storage Facilities 41. Textiles 42. Trade & Commerce 43. Transport 44. Tourism 45. Vital Statistics 46. Voluntary Services 47. Waterworks & Supply 48. Rubber Study DEPUTY DIRECTOR OF STATISTICS KANNIYAKUMARI DISTRICT PREFACE The District Statistical Hand Book is prepared and published by our Department every year. This book provides useful data across various departments in Kanniyakumari District. It contains imperative and essential statistical data on different Socio-Economic aspects of the District in terms of statistical tables and graphical representations. This will be useful in getting a picture of Kanniyakumari’s current state and analyzing what improvements can be brought further. I would liketo thank the respectable District Collector Sh. SAJJANSINGH R CHAVAN, IAS for his cooperation in achieving the task of preparing the District Hand Book for the year 2015-16 and I humbly acknowledge his support with profound gratitude. The co-operation extended by the officers of this district, by supplying the information presented in this book is gratefully acknowledged. -
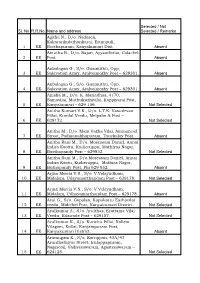
Selected / Not Sl
Selected / Not Sl. No R.R.No Name and address Selected / Remarks Agitha N., D/o. Nadasen, Kakarankulathankarai, Erumpuli, 1 EE Reethapuram, Kanyakumari Dist. Absent Amutha R., D/o. Rajan, Ayyandivilai, Colachel 2 EE Post. Absent Anbalagan G., S/o. Gnamuthu, Opp, 3 EE Salavation Army, Aralvoimozhy Post – 629301. Absent Anbalagan G., S/o. Gnamuthu, Opp, 4 EE Salavation Army, Aralvoimozhy Post – 629301. Absent Anisha M., D/o. K. Mariadhas, 4/70, Samavilai, Muthukuzhivilai, Kappiyarai Post, 5 EE Kanyakumari – 629 156. Not Selected Anitha Kumari V.K., D/o. L.T.K. Vasudevan Pillai, Kundal Veedu, Melpalai & Post – 6 EE 629152. Not Selected Anitha M., D/o. Mani Vazha Vilai, Ammancoil 7 EE Street, Padmanabhapuram, Thuckalay Post. Absent Anitha Rani M., D/o. Mosessam Daniel, Annai Indira Koottu, Kudieruppu, Mathiyas Nagar, 8 EE Boothapandy Post – 629852. Not Selected Anitha Rani M., D/o.Mosessam Daniel, Annai Indira Kootu, Kudieruppu, Mathias Nagar, 9 EE Buthapandy Post, Pin 629 852. Absent Arjun Morris V.S., S/o. V.Velayudham, 10 EE Midalam, Udayamarthandam Post – 629178. Not Selected Arjun Morris V.S., S/o. V.Velayudham, 11 EE Midalam, Udhayamarthandam Post – 629178. Absent Arul G., S/o. Gopalan, Kapukattu Eathavilai 12 EE veedu, Midichel Post, Kanyakumari District. Not Selected Arulkumar A., S/o. Arulthas, Erattama Vilai 13 EE Veedu, Edaicode Post – 629157. Not Selected Arulkumar K., S/o. Kunchu Pillai, Kollem Vilagam, Kollai, Kanjampuram Post, 14 EE Kanyakumari District. Absent Arumugam K., S/o. Karuppan, 43A/43 Arunthathiyar Street, Irulappapuram, Nagercoil, Vadiveeswaram, Agasteeswaram – 15 EE 624125. Not Selected Asha T., W/o. Murugesh, 27/17, East Street, 16 EE Thuvarangadu, Bhoothapandy Post – 629852. -

Kanyakumari District
Kanyakumari District Statistical Handbook 2010-11 1. Area & Population 2. Climate & Rainfall 3. Agriculture 4. Irrigation 5. Animal Husbandary 6. Banking & Insurance 7. Co-operation 8. Civil Supplies 9. Communications 10. Electricity 11. Education 12. Fisheries 13 Handloom 14. handicrafts 15. Health & Family Welfare 16. Housing 17. Industries 18. Factories 19. Legal Bodies 20. Labour&Employment 21. Legal Services 22. Libraries 23. Mining & Quarrying 24. Manufacturing 25. Medical services 26 Motor Vehicles 27. NonConventional Energy 28. Police & Prison 29. Public Health 30. Printing & publication 31. Price Indices. 32. Quality Control 33. Registration 34. Repair & Services 35. Restaurents & Hotels 36. Recreation 37. Social Welfare 38. Sanitary services 39. Scientific Research 40. Storage Facilities 41 Textiles 42. Trade & Commerce 43. Transport 44. Tourism 45. Birth & Death 46.Voluntary Services 47. Waterworks & Supply 1 1.AREA AND POPULATION 1.1 AREA, POPULATION, LITERATES, SC, ST – SEXWISE BY BLOCKS YEAR: 2010-2011 Population Literate Name of the Blocks/ Sl.No. Municipalities Male Male Female Female Persons Persons Area (sq.km) 1 2 3 4 5 6 7 8 9 1 Agastheswaram 133.12 148419 73260 75159 118778 60120 58658 2 Rajakkamangalam 120.16 137254 68119 69135 108539 55337 53202 3 Thovalai 369.07 110719 55057 55662 85132 44101 41031 4 Kurunthancode 106.85 165070 81823 83247 126882 64369 62513 5 Thuckalay 130.33 167262 82488 84774 131428 66461 64967 6 Thiruvattar 344.8 161619 80220 81399 122710 62524 60186 7 Killiyoor 82.7 156387 78663 77724 119931 -
![Kanyakumari District [ Revised Fee Structure for Appealed Schools ]](https://docslib.b-cdn.net/cover/5521/kanyakumari-district-revised-fee-structure-for-appealed-schools-2875521.webp)
Kanyakumari District [ Revised Fee Structure for Appealed Schools ]
01 - KANYAKUMARI DISTRICT [ REVISED FEE STRUCTURE FOR APPEALED SCHOOLS ] S.No C.Code School Name LKG UKG I II III IV V VI VII VIII IX X XI XII ALDRIN NURSERY & PRIMARY SCHOOL, 1 01003 1900 1900 1950 1950 1950 1950 1950 - - - - - - - KANDANVILAI- 629810. ANNAI CHELLAMMAL NURSERY & PRIMARY SCHOOL, 2 01004 1900 1900 2900 2900 2900 2900 2900 - - - - - - - 139,KEEZHOOR, THIRUPPATHISARAM. ARAMBOLY NURSERY & PRIMARY SCHOOL, 3 01005 NF NF NF NF NF NF NF NF NF NF NF NF NF NF ARALVAIMOZHI. ARULJOTHI NURSERY & PRIMARY SCHOOL, 4 01008 NF NF NF NF NF NF NF NF NF NF NF NF NF NF SOLAPURAM, KUMARAPURAM-629189. ARULMIGU PASUPATHEESWARAR NURSERY & PRIMARY 5 01009 SCHOOL, 1100 1100 1400 1400 1400 1400 1400 - - - - - - - IRULAPPAPURAM, NAGERCOIL. ARUNACHALA NURSERY & PRIMARY SCHOOL, 6 01010 2000 2000 2650 2650 2650 2650 2650 - - - - - - - MANAVILAI, VELLICHANTHAI PO, KANYAKUMARI DIST. ARUNODHAYA VIDYALAYA NURSERY & PRIMARY SCHOOL, 7 01011 3250 3250 3300 3300 3300 3300 3300 - - - - - - - MANJALAMOODU, EDAICODE - 629151. B.R MEMORIAL NURSERY & PRIMARY SCHOOL, 8 01012 1850 1850 2000 2000 2000 2000 2000 - - - - - - - PALUKAL, K.K.DIST. BELFIELD MATRICULATION HIGHER SECONDARY SCHOOL, ANANTHAN NAGAR, ASARIPALLAM POST - 629 201. 9 01014 4850 4850 4250 4250 4250 4250 4250 4700 4700 4700 5350 5350 6900 6900 KANYAKUMARI DISTRICT. Note:- NF - There is no Recognition, Hence No Fee is Fixed. 01 - KANYAKUMARI DISTRICT [ REVISED FEE STRUCTURE FOR APPEALED SCHOOLS ] S.No C.Code School Name LKG UKG I II III IV V VI VII VIII IX X XI XII BRITISH NURSERY & PRIMARY SCHOOL, 10 01018 53/25 CHANTHI ST, EDALAKUDY, 2600 2600 2050 2050 2050 2050 2050 - - - - - - - KOTTAR, NAGERCOIL - 629002. -

VI. the Seven and Half Churches St. Thomas Founded
VI. The Seven and Half Churches St. Thomas Founded As we have seen already, the Ezharapallikal (seven and half churhces) that St. Thomas the Apostle founded are at Kottakkayal (Kottakkavu. Present day North Paravur) Maliankara (Kodungallur, Crangannoor), Palayoor, Kollam, Niranam, Nilackal (Chayal), Kokkamangalam and Thiruvithamcode (Arapally, that is, Half-Church). 1. Kottakkayal or Kottakavu (Muziris) St. Thomas landed in Muziris, which was a big harbour, and in this geographical area, St. Thomas founded two Christian communities one at Kottakayal (Kottakkavu) and the other at Maliankara (Kodungallur- Azhikode). (picture 5) The history of Muziris starts from early 3000 BC when Babylonians, Assyrians and Egyptians came to the Malabar Coast (Kerala) in search for the spices. Later these Middle-East groups were joined by Arabs and Phoenicians. And gradually Muziris entered into the cartography of World trade map. Then onwards Muziris holds the key to a good chunk of Kerala's ancient history now the ancient trade route. The port was a key to the trade between southern India and the Phoenicians, the Persians, the Egyptians, the Greeks and the Roman Empire. The important known commodities exported from Muziris were spices (such as black pepper and malabathron, that is, cinnamon), semi-precious stones (such as beryl including emerald), pearls, diamonds, sapphires, ivory, silk, Gangetic spikenard and tortoise shells. The Romans brought money (in gold coins), peridots, thin clothing, figured linens, multicoloured textiles, sulfide of antimony, copper, tin, lead, coral, raw glass, wine, realgar and orpiment. Muziris has found mention in the bardic Tamil Sangam literature (300 BC to AD 300) and a number of classical European historical sources. -

Union Bank of India, Regional Office, P.T. Rajan Road, Madurai – 625 002
Appendix See proviso to rule 8 (6) of The Security Interest (Enforcement) Rules, 2002. Residential Property with land admeasuring 5 cents at Survey No.795/6, Door No.16-105A, Chempilavilai, Thickanamcode Village, Kalukulam Taluk, Kanyakumari District. E- Auction Sale Notice for sale of immovable assets under the Securitization and Reconstruction of Financial Assets and Enforcement of Security Interest Act, 2002 read with proviso to Rule 8 (6) of the Security Interest (Enforcement) Rules 2002. Notice is hereby given to the public in general and in particular to the borrower(s) and Guarantor (s) that the below described immovable property mortgaged/ charged to the Secured Creditor, the physical possession of which has been taken by the Authorized Officer of Union Bank of India, eCB-Karungal Branch the Secured Creditor, will be sold on “As is Where is”, “As is what is”, and “whatever there is” on 15/10/2020 for recovery of Rs. 10,26,439.80 in A/c. No. 560631001888675 as of 30/08/2020 with further interest thereon and other charges due to the Secured Creditor from Mr. Raja & Ms. Shanthi Account with Union Bank of India, eCB-Karungal Branch. Description of the property Land with residential building built thereon located at Survey No. 795/6, Door No. 16-105A, Chempilavilai, Thickanamcode Village(Formerly Thiruvithamcode Village), Kalkulam Taluk, Kanyakumari District owned by Mr.Raja and Ms.Shanthi. Total extent of land – 5 cents and plinth area of building – 1096 sq.ft. Boundaries: East : Primary School South : Primary School, Property belonged to Paulaiyan West : Road North : Property belonged to Shanthi The reserve price will be Rs.14,98,000.00 and the Earnest money deposit will be Rs. -

MARTHANDAVARMA – the Legend of Modern Travancore
International Journal of Research ISSN NO:2236-6124 MARTHANDAVARMA – The Legend of Modern Travancore Sharmila Prasad R. Ph.D. Research Scholar (Reg. No.1035/2014) Research Department of History, Alagappa Government Arts College, Karaikkudi – 630 003. Tamil Nadu, India. (Affiliated to Alagappa University, Karaikudi – 630003, Tamil Nadu, India.) Dr. C. Lawrance Assistant Professor (Research Supervisor) Research Department of History, Alagappa Government Arts College, Karaikkudi – 630 003. Tamil Nadu, India. (Affiliated to Alagappa University, Karaikudi – 630003, Tamil Nadu, India.) ----------------------------- ABSTRACT The Travancore, erstwhile Hindu feudal kingdom, is one of the most scenic and charming portions of India. The term Travancore is the anglicized form of Thiruvithamkodu which means 'the abode of prosperity'. It derived its name from the word Thiruvithamcode, its one- time capital. Anizham Thirunal Marthandavarma was the King of Travancore from 1729 A.D until his death in 1758 A.D. The accession of Marthandavarma to the throne marked the commencement of a new era in the annals of the administrative past of Travancore. Within a few years of his accession, he was able to put down the over mighty subjects and restore peace and order throughout his country. Later, he waged continuous wars against several of his northern neighbours and conquered them. These struggles with his enemies did not prevent him from establishing an effective administration and undertaking several nation-building activities. King Marthandavarma was the only Indian king to beat the European army at the Battle of Colachel against the Dutch. For administrative convenience, Marthandavarma re-organised all departments. He followed Blood and Iron policy in uniting the Travancore kingdom. -

Downloaded for Personal Non-Commercial Research Or Study, Without Prior Permission Or Charge
R Knight, Sarah (2019) Narratives of religious identity: the self-perception of the Jacobite Syrian Christians of Kerala. https://eprints.soas.ac.uk/34855/ Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this thesis, full bibliographic details including the author, title, awarding institution and date of the thesis must be given e.g. AUTHOR (year of submission) "Full thesis title", name of the School or Department, PhD Thesis, pagination. Narratives of religious identity: the self-perception of the Jacobite Syrian Christians of Kerala Volume I Sarah Knight Thesis submitted for the Degree of PhD in the Study of Religions 2019 Department of the Study of Religions and Philosophies School of Oriental and African Studies University of London Abstract This thesis examines the question of the religious self-definition of the Jacobite Syrian Christian community in Kerala. The leading question is: to what extent does the indigenous narrative of that community about their religious identity differ from existing dominant historical accounts? It examines texts in Malayalam from the Jacobite Syrian Christians, particularly the unpublished 18th century Mathai Vettikkunnel manuscript, in order to investigate the narrative of their religious identity, in the context of existing scholarly discourse.