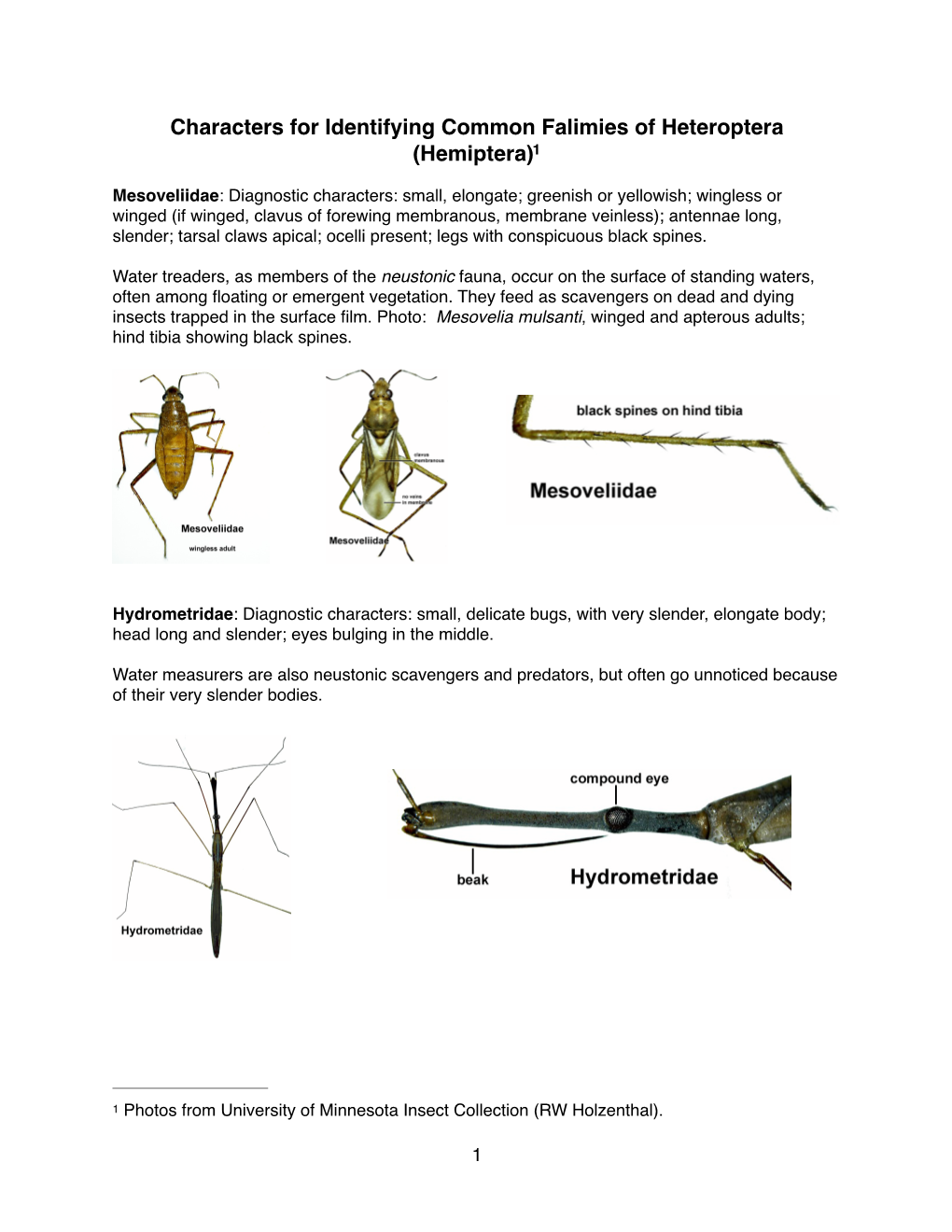
Characters for Identifying Common Falimies of Heteroptera (Hemiptera)1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morphology and Adaptation of Immature Stages of Hemipteran Insects
© 2019 JETIR January 2019, Volume 6, Issue 1 www.jetir.org (ISSN-2349-5162) Morphology and Adaptation of Immature Stages of Hemipteran Insects Devina Seram and Yendrembam K Devi Assistant Professor, School of Agriculture, Lovely Professional University, Phagwara, Punjab Introduction Insect Adaptations An adaptation is an environmental change so an insect can better fit in and have a better chance of living. Insects are modified in many ways according to their environment. Insects can have adapted legs, mouthparts, body shapes, etc. which makes them easier to survive in the environment that they live in and these adaptations also help them get away from predators and other natural enemies. Here are some adaptations in the immature stages of important families of Hemiptera. Hemiptera are hemimetabolous exopterygotes with only egg and nymphal immature stages and are divided into two sub-orders, homoptera and heteroptera. The immature stages of homopteran families include Delphacidae, Fulgoridae, Cercopidae, Cicadidae, Membracidae, Cicadellidae, Psyllidae, Aleyrodidae, Aphididae, Phylloxeridae, Coccidae, Pseudococcidae, Diaspididae and heteropteran families Notonectidae, Corixidae, Belastomatidae, Nepidae, Hydrometridae, Gerridae, Veliidae, Cimicidae, Reduviidae, Pentatomidae, Lygaeidae, Coreidae, Tingitidae, Miridae will be discussed. Homopteran families 1. Delphacidae – Eg. plant hoppers They comprise the largest family of plant hoppers and are characterized by the presence of large, flattened spurs at the apex of their hind tibiae. Eggs are deposited inside plant tissues, elliptical in shape, colourless to whitish. Nymphs are similar in appearance to adults except for size, colour, under- developed wing pads and genitalia. 2. Fulgoridae – Eg. lantern bugs They can be recognized with their antennae inserted on the sides & beneath the eyes. -

Insetos Do Brasil
COSTA LIMA INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 INSETOS DO BRASIL 2.º TOMO HEMÍPTEROS A. DA COSTA LIMA Professor Catedrático de Entomologia Agrícola da Escola Nacional de Agronomia Ex-Chefe de Laboratório do Instituto Oswaldo Cruz INSETOS DO BRASIL 2.º TOMO CAPÍTULO XXII HEMÍPTEROS ESCOLA NACIONAL DE AGRONOMIA SÉRIE DIDÁTICA N.º 3 - 1940 CONTEUDO CAPÍTULO XXII PÁGINA Ordem HEMÍPTERA ................................................................................................................................................ 3 Superfamília SCUTELLEROIDEA ............................................................................................................ 42 Superfamília COREOIDEA ............................................................................................................................... 79 Super família LYGAEOIDEA ................................................................................................................................. 97 Superfamília THAUMASTOTHERIOIDEA ............................................................................................... 124 Superfamília ARADOIDEA ................................................................................................................................... 125 Superfamília TINGITOIDEA .................................................................................................................................... 132 Superfamília REDUVIOIDEA ........................................................................................................................... -

Review of Acanthocephala (Hemiptera: Heteroptera: Coreidae) of America North of Mexico with a Key to Species
Zootaxa 2835: 30–40 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Review of Acanthocephala (Hemiptera: Heteroptera: Coreidae) of America north of Mexico with a key to species J. E. McPHERSON1, RICHARD J. PACKAUSKAS2, ROBERT W. SITES3, STEVEN J. TAYLOR4, C. SCOTT BUNDY5, JEFFREY D. BRADSHAW6 & PAULA LEVIN MITCHELL7 1Department of Zoology, Southern Illinois University, Carbondale, Illinois 62901, USA. E-mail: [email protected] 2Department of Biological Sciences, Fort Hays State University, Hays, Kansas 67601, USA. E-mail: [email protected] 3Enns Entomology Museum, Division of Plant Sciences, University of Missouri, Columbia, Missouri 65211, USA. E-mail: [email protected] 4Illinois Natural History Survey, University of Illinois at Urbana-Champaign, Illinois 61820, USA. E-mail: [email protected] 5Department of Entomology, Plant Pathology, & Weed Science, New Mexico State University, Las Cruces, New Mexico 88003, USA. E-mail: [email protected] 6Department of Entomology, University of Nebraska-Lincoln, Panhandle Research & Extension Center, Scottsbluff, Nebraska 69361, USA. E-mail: [email protected] 7Department of Biology, Winthrop University, Rock Hill, South Carolina 29733, USA. E-mail: [email protected] Abstract A review of Acanthocephala of America north of Mexico is presented with an updated key to species. A. confraterna is considered a junior synonym of A. terminalis, thus reducing the number of known species in this region from five to four. New state and country records are presented. Key words: Coreidae, Coreinae, Acanthocephalini, Acanthocephala, North America, review, synonymy, key, distribution Introduction The genus Acanthocephala Laporte currently is represented in America north of Mexico by five species: Acan- thocephala (Acanthocephala) declivis (Say), A. -

Does Argentine Ant Invasion Conserve Colouring Variation of Myrmecomorphic Jumping Spider?
Open Journal of Animal Sciences, 2014, 4, 144-151 Published Online June 2014 in SciRes. http://www.scirp.org/journal/ojas http://dx.doi.org/10.4236/ojas.2014.43019 Argentine Ant Affects Ant-Mimetic Arthropods: Does Argentine Ant Invasion Conserve Colouring Variation of Myrmecomorphic Jumping Spider? Yoshifumi Touyama1, Fuminori Ito2 1Niho, Minami-ku, Hiroshima City, Japan 2Laboratory of Entomology, Faculty of Agriculture, Kagawa University, Ikenobe, Japan Email: [email protected] Received 23 April 2014; revised 3 June 2014; accepted 22 June 2014 Copyright © 2014 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Argentine ant invasion changed colour-polymorphic composition of ant-mimetic jumping spider Myrmarachne in southwestern Japan. In Argentine ant-free sites, most of Myrmarachne exhibited all-blackish colouration. In Argentine ant-infested sites, on the other hand, blackish morph de- creased, and bicoloured (i.e. partly bright-coloured) morphs increased in dominance. Invasive Argentine ant drives away native blackish ants. Disappearance of blackish model ants supposedly led to malfunction of Batesian mimicry of Myrmarachne. Keywords Batesian Mimicry, Biological Invasion, Linepithema humile, Myrmecomorphy, Myrmarachne, Polymorphism 1. Introduction It has attracted attention of biologists that many arthropods morphologically and/or behaviorally resemble ants [1]-[4]. Resemblance of non-ant arthropods to aggressive and/or unpalatable ants is called myrmecomorphy (ant-mimicry). Especially, spider myrmecomorphy has been described through many literatures [5]-[9]. Myr- mecomorphy is considered to be an example of Batesian mimicry gaining protection from predators. -

Hemiptera: Heteroptera) of Magallanes Region: Checklist and Identification Key to the Species
Anales Instituto Patagonia (Chile), 2016. Vol. 44(1):39-42 39 The Coreoidea Leach, 1815 (Hemiptera: Heteroptera) of Magallanes Region: Checklist and identification key to the species Los Coreoidea Leach, 1815 (Hemiptera: Heteroptera) de la Región de Magallanes: Lista de especies y clave de identificación Eduardo I. Faúndez1,2 Abstract Slater, 1995), and several species are economically Members of the Coreoidea of Magallanes Region important; there are, however, also cases in which are listed. First records in the Magallanes Region are species of this superfamily have been recorded provided for Harmostes (Neoharmostes) procerus feeding on carrion and dung (Mitchell, 2000). Berg, 1878 and Althos nigropunctatus (Signoret, Additionally, biting humans has been recorded 1864). It is concluded that three species classified in members of this group (Faúndez & Carvajal, in three genera and two families are present in the 2011). In Chile, the Coreoidea is represented by region. A key to the species is provided. two families, the Coreidae and Rhopalidae, and the major diversity for this group is found in the central Key words: Coreidae, Rhopalidae, Distribution, zone of the country (Faúndez, 2015b). New records, Chile. In Magallanes, very little is known about the species of this superfamily, and actually there is Resumen only one species officially recorded from the area: Se listan los Coreoidea de la Region de Magallanes. the dunes bug, Eldarca nigroscutellata Faúndez, Se entregan los primeros registros para la región 2015 (Coreidae). The purpose of this contribution de Harmostes (Neoharmostes) procerus Berg, is to provide an update of this group in the 1878 y Althos nigropunctatus (Signoret, 1864). -

The Corixidae (Hemiptera) of Oklahoma KURT F
BIOLOGICAL SCIENCES 71 The Corixidae (Hemiptera) of Oklahoma KURT F. SCHAEFER, Panhandle State Colle.e, Goodwell The Corixidae or water boatman family is a commonly collected fam ily taken in a variety of aquatic habitats and frequently at lights at night or on shiny surfaces during the day. Hungerford's 1948 monograph on the world corixids is an important contribution, essential to a serious collector. My paper is an attempt to make the identification of state fonns easier and to supply descriptions and distribution data for the corixids of the state. Schaefer and Drew (1964) reported 18 species and Ewing (1964) added one for the state. Five addi tional species are included because information of their known ranges in dicates that they will probably be found in Oklahoma when more collecting is done. Each pair of legs is modified for a different function. The anterior pair is short with the tenninal segment (pala) often more or less spoon shaped and fringed with bristles for food gathering. Both adults and nymphs feed mainly on algae and protozoa, obtained from bottom ooze (Usinger, 1956). The middle pair of legs, used for anchorage and support, is long and slender, tenninating with two long claws. The hind pair, for swimming, is stouter, laterally flattened and fringed with hairs. The principal dimorphic structures used as key characters are as fol lows: males, usually smaller, with vertex of the head otten more produced and frons concavely depressed. Fonn and chaetotaxy of the male palae, front tarsi, are much used characters. The female abdomen is bilaterally symmetrical, while the asymmetry of male may be either to the right (dextral) or left (stnistral). -

Incidence of Rice Bug,Leptocorisaoratorius (F.) (Hemiptera: Alydidae) Using White Muscardinefungus Beauveriabassiana (Bals.) Vuill.In Upland Rice
IJISET - International Journal of Innovative Science, Engineering & Technology, Vol. 1 Issue 10, December 2014. www.ijiset.com ISSN 2348 – 7968 Incidence of Rice Bug,Leptocorisaoratorius (F.) (Hemiptera: Alydidae) Using White MuscardineFungus Beauveriabassiana (Bals.) Vuill.In Upland Rice Pio P. Tuan, PhD* Department of Agricultural Sciences, College of Agriculture, Fisheries, and Natural Resources University of Eastern Philippines, University Town, Catarman, Northern Samar, Philippines Abstract A field experiment was conducted to evaluate the incidence of Rice bug, Leptocorisaoratorius(F.) using B. bassiana as mycoinsectice under upland conditions. Field population of L. oratorius was not significantly affected by B. bassiana7 DAS, but significant at 15 DAS. The application of B. bassiana did not significantly affect the damage caused by L. oratoriuson rice grains.The results suggest that B. bassiana cannot be used as a sole mortality factor in the management of rice bug under upland conditions. More field experimentations are necessary taking into consideration the influence of environmental factors and how these can be manipulated in favor of the fungal insecticide. Key Words: Conidia, substrate, microbial insecticide, upand rice, abiotic environmental factors INTRODUCTION Rice bug, Leptrocorisaoratorius (F.) is one of the major insect pests infesting rice in upland areas. Several species of rice bugs occur in the Philippines, but L. oratorius is the most prevalent (Reissig et al., 1986; Litsinger et al., 1987). Upland rice is usually cultivated for organic rice or with less application of fertilizer and pesticides. The increasing demand for organically produced foods including rice, has contributed to the adoption of ecologically oriented pest control methods. Consequently, reduced pesticide use has become a strong option to protect the environment and human health. -

An Overview of Flat Bug Genera (Hemiptera, Aradidae)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Denisia Jahr/Year: 2006 Band/Volume: 0019 Autor(en)/Author(s): Lariviere Marie C., Larochelle Andre Artikel/Article: An overview of flat bug genera (Hemiptera, Aradidae) from New Zealand, with considerations on faunal diversification and affinities 181-214 © Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at An overview of flat bug genera (Hemiptera, Aradidae) from New Zealand, with considerations on faunal diversification and affinities1 M .-C . L ARIVIÈRE & A. L AROCHELLE Abstract: Nineteen genera and thirty-nine species of Aradidae have been described from New Zealand, most of which are endemic (12 genera, 38 species). An overview of all genera and an identification key to subfamilies, tribes, and genera are presented for the first time. Species included in each genus are list- ed for New Zealand. Concise generic descriptions, illustrations emphasizing key diagnostic features, colour photographs representing each genus, an overview of the most relevant literature, and notes on distribution are also given. The biology and diversification of New Zealand aradids, and their affinities with neighbouring faunas are briefly discussed. Key words: Aradidae, biogeography, Hemiptera, New Zealand, taxonomy. Introduction forests, and using their stylets to extract liq- uids from fungal hyphae associated with de- The Aradidae, also commonly referred caying wood. Many ground-dwelling species to as flat bugs or bark bugs, form a large fam- of rainforest environments are wingless – a ily of Heteroptera containing over 1,800 condition thought to have evolved several species and 210 genera worldwide (SCHUH times in the phylogeographic history of the & SLATER 1995). -

The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V
The Semiaquatic Hemiptera of Minnesota (Hemiptera: Heteroptera) Donald V. Bennett Edwin F. Cook Technical Bulletin 332-1981 Agricultural Experiment Station University of Minnesota St. Paul, Minnesota 55108 CONTENTS PAGE Introduction ...................................3 Key to Adults of Nearctic Families of Semiaquatic Hemiptera ................... 6 Family Saldidae-Shore Bugs ............... 7 Family Mesoveliidae-Water Treaders .......18 Family Hebridae-Velvet Water Bugs .......20 Family Hydrometridae-Marsh Treaders, Water Measurers ...22 Family Veliidae-Small Water striders, Rime bugs ................24 Family Gerridae-Water striders, Pond skaters, Wherry men .....29 Family Ochteridae-Velvety Shore Bugs ....35 Family Gelastocoridae-Toad Bugs ..........36 Literature Cited ..............................37 Figures ......................................44 Maps .........................................55 Index to Scientific Names ....................59 Acknowledgement Sincere appreciation is expressed to the following individuals: R. T. Schuh, for being extremely helpful in reviewing the section on Saldidae, lending specimens, and allowing use of his illustrations of Saldidae; C. L. Smith for reading the section on Veliidae, checking identifications, and advising on problems in the taxon omy ofthe Veliidae; D. M. Calabrese, for reviewing the section on the Gerridae and making helpful sugges tions; J. T. Polhemus, for advising on taxonomic prob lems and checking identifications for several families; C. W. Schaefer, for providing advice and editorial com ment; Y. A. Popov, for sending a copy ofhis book on the Nepomorpha; and M. C. Parsons, for supplying its English translation. The University of Minnesota, including the Agricultural Experi ment Station, is committed to the policy that all persons shall have equal access to its programs, facilities, and employment without regard to race, creed, color, sex, national origin, or handicap. The information given in this publication is for educational purposes only. -

Insects of Larose Forest (Excluding Lepidoptera and Odonates)
Insects of Larose Forest (Excluding Lepidoptera and Odonates) • Non-native species indicated by an asterisk* • Species in red are new for the region EPHEMEROPTERA Mayflies Baetidae Small Minnow Mayflies Baetidae sp. Small minnow mayfly Caenidae Small Squaregills Caenidae sp. Small squaregill Ephemerellidae Spiny Crawlers Ephemerellidae sp. Spiny crawler Heptageniiidae Flatheaded Mayflies Heptageniidae sp. Flatheaded mayfly Leptophlebiidae Pronggills Leptophlebiidae sp. Pronggill PLECOPTERA Stoneflies Perlodidae Perlodid Stoneflies Perlodid sp. Perlodid stonefly ORTHOPTERA Grasshoppers, Crickets and Katydids Gryllidae Crickets Gryllus pennsylvanicus Field cricket Oecanthus sp. Tree cricket Tettigoniidae Katydids Amblycorypha oblongifolia Angular-winged katydid Conocephalus nigropleurum Black-sided meadow katydid Microcentrum sp. Leaf katydid Scudderia sp. Bush katydid HEMIPTERA True Bugs Acanthosomatidae Parent Bugs Elasmostethus cruciatus Red-crossed stink bug Elasmucha lateralis Parent bug Alydidae Broad-headed Bugs Alydus sp. Broad-headed bug Protenor sp. Broad-headed bug Aphididae Aphids Aphis nerii Oleander aphid* Paraprociphilus tesselatus Woolly alder aphid Cicadidae Cicadas Tibicen sp. Cicada Cicadellidae Leafhoppers Cicadellidae sp. Leafhopper Coelidia olitoria Leafhopper Cuernia striata Leahopper Draeculacephala zeae Leafhopper Graphocephala coccinea Leafhopper Idiodonus kelmcottii Leafhopper Neokolla hieroglyphica Leafhopper 1 Penthimia americana Leafhopper Tylozygus bifidus Leafhopper Cercopidae Spittlebugs Aphrophora cribrata -

Broad-Headed Bugs (Alydidae)
Chapter 18 Broad-Headed Bugs (Alydidae) Antônio R. Panizzi and Carl w. Schaefer Abstract The broad-headed bugs (Alydidae) are divided into two subfamilies, Alydinaeand Micrelytrinae, each divided into two tribes, Daclerini and Alydini, and Micrelytriniand Leptocorisini, respectively, The farnily has 53 genera and about 250 specieins; the Neotropics, there are 21 genera. Alydids are small (8-20 mm), slen- Itr,with a triangular head; nymphs of alydines mimic ants, the adults of some Micrelytrinialso rnirnic ants. The most studied species in the Neotropics is the aly- dineNeomegalotomus parvus (Westwood), usually associated with legumes, and maybe a pest on soybean. Other common genera include Hyalymenus Amyot & Serville,Stenocoris Burmeister, Cydamus Stâl, and Trachelium Herrich-Schâffer. Studieson taxonomy and bioecology on alydids of the Neotropics are needed. 18.1 Introduction AlydidaeAmyot and Serville, 1843, were treated as a subfarnily of the farnily Coreidaeand even as a tribe (Schaffner 1964); now it has been treated as a farnily, ~ether with Coreidae, Rhopalidae, Hyocephalidae, and Stenocephalidae, in the !UperfarniCoreoidealy (Schaefer 1964). Thisfarnily contains 53 genera and approximately 250 species, mostly tropical Irsubtropical,in all regions of the world. There are only two genera that span both dleOldand the New World, Alydus and Megalotomus. These genera are Holarctic, IInAlydus extends from Alaska through Canada into Mexico (Brailovsky and Flores 1979;Froeschner 1988; Maw et al. 2000). The genera of Alydinae have been revised by Schaffner (1964; 22 species worldwide);the world genera of the subfamily Micrelytrinae, tribe Leptocorisini, were CarlW.Schaefer: Author deceased at the time of publication A.RP.anizzi ([gJ) Laboratóriode Entomologia, Embrapa Trigo, Caixa Postal 3081, Passo Fundo, RS9900l-970,Brazil e-mail:[email protected] eSpringerScience-Business Media Dordrecht 2015 537 :I.R.Panizzi,J. -

Heteroptera: Miridae, Orthotylinae) Adam Asquith Systematic Entomology Laboratory, Department of Entomology, Oregon State University, Corvallis
Great Basin Naturalist Volume 50 | Number 2 Article 5 6-30-1990 Taxonomy and variation of the Lopidea nigridia complex of western North America (Heteroptera: Miridae, Orthotylinae) Adam Asquith Systematic Entomology Laboratory, Department of Entomology, Oregon State University, Corvallis Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Asquith, Adam (1990) "Taxonomy and variation of the Lopidea nigridia complex of western North America (Heteroptera: Miridae, Orthotylinae)," Great Basin Naturalist: Vol. 50 : No. 2 , Article 5. Available at: https://scholarsarchive.byu.edu/gbn/vol50/iss2/5 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Creal llQ~ln N"h,,-albl 50;2), 1900,1)11. l35-l54 TAXONOMY AND VARIATION OFTHE LOPIDEA NIGRIDIA COMPLEX OF WESTERN NORTH AMERICA (HETEROPTERA: MIRIDAE: ORTHOTYUNAE) Adam Asquith: ARSTRACT.-External morphological variation ill the topMen 1Jigridin "complex" of western North America wa<; examined using principal component analysis and showed c.."Ontintious variation amon~ populalions in must (·haracters. Extenml morphologydid nol parallel paramcrc structure ;\lId did not substantiate previously recognized species. There was littlecorrelation between dorsal coloration and (xtr·.amcre structure. Clustcr,Ulalysis (UPCMA) using paramert and cHlor characters fuik..d to group populations etXIed as the samc species and also failed to group aU specimens ofany one pupulatinn, The variation in structure ofthe pilr.lmerCS anu vesicae among popul<1tions ofthe Iligtidi(l complex wall no greater than the interpopulationaJ variation ofthese structures in the c()ll~encricspecies mflTgirwta Uhler.