Appendices a & B
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Seriously Playful: Philosophy in the Myths of Ovid's Metamorphoses
Seriously Playful: Philosophy in the Myths of Ovid’s Metamorphoses Megan Beasley, B.A. (Hons) This thesis is presented for the degree of Doctor of Philosophy of Classics of The University of Western Australia School of Humanities Discipline of Classics and Ancient History 2012 1 2 For my parents 3 4 Abstract This thesis aims to lay to rest arguments about whether Ovid is or is not a philosophical poet in the Metamorphoses. It does so by differentiating between philosophical poets and poetic philosophers; the former write poetry freighted with philosophical discourse while the latter write philosophy in a poetic medium. Ovid, it is argued, should be categorised as a philosophical poet, who infuses philosophical ideas from various schools into the Metamorphoses, producing a poem that, all told, neither expounds nor attacks any given philosophical school, but rather uses philosophy to imbue its constituent myths with greater wit, poignancy and psychological realism. Myth and philosophy are interwoven so intricately that it is impossible to separate them without doing violence to Ovid’s poem. It is not argued here that the Metamorphoses is a fundamentally serious poem which is enhanced, or marred, by occasional playfulness. Nor is it argued that the poem is fundamentally playful with occasional moments of dignity and high seriousness. Rather, the approach taken here assumes that seriousness and playfulness are so closely connected in the Metamorphoses that they are in fact the same thing. Four major myths from the Metamorphoses are studied here, from structurally significant points in the poem. The “Cosmogony” and the “Speech of Pythagoras” at the beginning and end of the poem have long been recognised as drawing on philosophy, and discussion of these two myths forms the beginning and end of the thesis. -

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida
Nova Southeastern University NSUWorks HCNSO Student Capstones HCNSO Student Work 4-28-2020 A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida Anne C. Sevon Nova Southeastern University, [email protected] This document is a product of extensive research conducted at the Nova Southeastern University . For more information on research and degree programs at the NSU , please click here. Follow this and additional works at: https://nsuworks.nova.edu/cnso_stucap Part of the Marine Biology Commons, and the Oceanography and Atmospheric Sciences and Meteorology Commons Share Feedback About This Item NSUWorks Citation Anne C. Sevon. 2020. A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida. Capstone. Nova Southeastern University. Retrieved from NSUWorks, . (350) https://nsuworks.nova.edu/cnso_stucap/350. This Capstone is brought to you by the HCNSO Student Work at NSUWorks. It has been accepted for inclusion in HCNSO Student Capstones by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Capstone of Anne C. Sevon Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science M.S. Marine Environmental Sciences M.S. Coastal Zone Management Nova Southeastern University Halmos College of Natural Sciences and Oceanography April 2020 Approved: Capstone Committee Major Professor: Dr. Kirk Kilfoyle Committee Member: Dr. Bernhard Riegl This capstone is available at NSUWorks: https://nsuworks.nova.edu/cnso_stucap/350 HALMOS -

Observations on Neritina Turrita (Gmelin 1791) Breeding Behaviour in Laboratory Conditions
Hristov, K.K. AvailableInd. J. Pure online App. Biosci. at www.ijpab.com (2020) 8(5), 1-10 ISSN: 2582 – 2845 DOI: http://dx.doi.org/10.18782/2582-2845.8319 ISSN: 2582 – 2845 Ind. J. Pure App. Biosci. (2020) 8(5), 1-10 Research Article Peer-Reviewed, Refereed, Open Access Journal Observations on Neritina turrita (Gmelin 1791) Breeding Behaviour in Laboratory Conditions Kroum K. Hristov* Department of Chemistry and Biochemistry, Medical University - Sofia, Sofia - 1431, Bulgaria *Corresponding Author E-mail: [email protected] Received: 15.08.2020 | Revised: 22.09.2020 | Accepted: 24.09.2020 ABSTRACT Neritina turrita (Gmelin 1791) along with other Neritina, Clithon, Septaria, and other fresh- water snails are popular animals in ornamental aquarium trade. The need for laboratory-bred animals, eliminating the potential biohazard risks, for the ornamental aquarium trade and the growing demand for animal model systems for biomedical research reasons the work for optimising a successful breading protocol. The initial results demonstrate N. turrita as tough animals, surviving fluctuations in pH from 5 to 9, and shifts from a fresh-water environment to brackish (2 - 20 ppt), to sea-water (35 ppt) salinities. The females laid over 630 (at salinities 0, 2, 10 ppt and temperatures of 25 - 28oC) white oval 1 by 0.5 mm egg capsules continuously within 2 months after collecting semen from several males. Depositions of egg capsules are set apart 6 +/-3 days, and consist on average of 53 (range 3 to 192) egg capsules. Production of viable veligers was recorded under laboratory conditions. Keywords: Neritina turrita, Sea-water, Temperatures, Environment INTRODUCTION supposably different genera forming hybrids Neritininae are found in the coastal swamps of with each other, suggesting their close relation. -

Lucan's Natural Questions: Landscape and Geography in the Bellum Civile Laura Zientek a Dissertation Submitted in Partial Fulf
Lucan’s Natural Questions: Landscape and Geography in the Bellum Civile Laura Zientek A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2014 Reading Committee: Catherine Connors, Chair Alain Gowing Stephen Hinds Program Authorized to Offer Degree: Classics © Copyright 2014 Laura Zientek University of Washington Abstract Lucan’s Natural Questions: Landscape and Geography in the Bellum Civile Laura Zientek Chair of the Supervisory Committee: Professor Catherine Connors Department of Classics This dissertation is an analysis of the role of landscape and the natural world in Lucan’s Bellum Civile. I investigate digressions and excurses on mountains, rivers, and certain myths associated aetiologically with the land, and demonstrate how Stoic physics and cosmology – in particular the concepts of cosmic (dis)order, collapse, and conflagration – play a role in the way Lucan writes about the landscape in the context of a civil war poem. Building on previous analyses of the Bellum Civile that provide background on its literary context (Ahl, 1976), on Lucan’s poetic technique (Masters, 1992), and on landscape in Roman literature (Spencer, 2010), I approach Lucan’s depiction of the natural world by focusing on the mutual effect of humanity and landscape on each other. Thus, hardships posed by the land against characters like Caesar and Cato, gloomy and threatening atmospheres, and dangerous or unusual weather phenomena all have places in my study. I also explore how Lucan’s landscapes engage with the tropes of the locus amoenus or horridus (Schiesaro, 2006) and elements of the sublime (Day, 2013). -
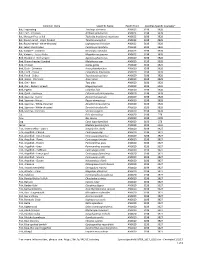
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -

NC-Anchovy-And-Identification-Key
Anchovy (Family Engraulidae) Diversity in North Carolina By the NCFishes.com Team Engraulidae is a small family comprising six species in North Carolina (Table 1). Their common name, anchovy, is possibly from the Spanish word anchova, but the term’s ultimate origin is unclear (https://en.wiktionary.org/wiki/anchovy, accessed December 18, 2020). North Carolina’s anchovies range in size from about 100 mm Total Length for Bay Anchovy and Cuban Anchovy to about 150 mm Total Length for Striped Anchovy (Munroe and Nizinski 2002). Table 1. Species of anchovies found in or along the coast of North Carolina. Scientific Name/ Scientific Name/ American Fisheries Society Accepted Common Name American Fisheries Society Accepted Common Name Engraulis eurystole - Silver Anchovy Anchoa mitchilli - Bay Anchovy Anchoa hepsetus - Striped Anchovy Anchoa cubana - Cuban Anchovy Anchoa lyolepis - Dusky Anchovy Anchoviella perfasciata - Flat Anchovy We are not aware of any other common names applied to this family, except for calling all of them anchovies. But as we have learned, each species has its own scientific (Latin) name which actually means something (please refer to The Meanings of the Scientific Names of Anchovies, page 9) along with an American Fisheries Society-accepted common name (Table 1; Page et al. 2013). Anchovies from large schools of fishes that feed on zooplankton. In North Carolina they may be found in all coastal basins, nearshore, and offshore (Tracy et al. 2020; NCFishes.com [Please note: Tracy et al. (2020) may be downloaded for free at: https://trace.tennessee.edu/sfcproceedings/vol1/iss60/1.] All species are found in saltwater environments (Maps 1-6), but Bay Anchovy is a seasonal freshwater inhabitant in our coastal rivers as far upstream as near Lock and Dam No. -
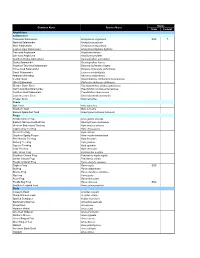
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Anchoa Mitchilli) Eggs and Larvae in Chesapeake Bay ) E.W
Estuarine, Coastal and Shelf Science 60 (2004) 409e429 www.elsevier.com/locate/ECSS Distribution and transport of bay anchovy (Anchoa mitchilli) eggs and larvae in Chesapeake Bay ) E.W. North , E.D. Houde1 University of Maryland Center for Environmental Science, Chesapeake Biological Laboratory, USA Received 28 February 2003; accepted 8 January 2004 Abstract Mechanisms and processes that influence small-scale depth distribution and dispersal of bay anchovy (Anchoa mitchilli) early-life stages are linked to physical and biological conditions and to larval developmental stage. A combination of fixed-station sampling, an axial abundance survey, and environmental monitoring data was used to determine how wind, currents, time of day, physics, developmental stage, and prey and predator abundances interacted to affect the distribution and potential transport of eggs and larvae. Wind-forced circulation patterns altered the depth-specific physical conditions at a fixed station and significantly influenced organism distributions and potential transport. The pycnocline was an important physical feature that structured the depth distribution of the planktonic community: most bay anchovy early-life stages (77%), ctenophores (72%), copepod nauplii (O76%), and Acartia tonsa copepodites (69%) occurred above it. In contrast, 90% of sciaenid eggs, tentatively weakfish (Cynoscion regalis), were found below the pycnocline in waters where dissolved oxygen concentrations were !2.0 mg lÿ1. The dayenight cycle also influenced organism abundances and distributions. Observed diel periodicity in concentrations of bay anchovy and sciaenid eggs, and of bay anchovy larvae O6 mm, probably were consequences of nighttime spawning (eggs) and net evasion during the day (larvae). Diel periodicity in bay anchovy swimbladder inflation also was observed, indicating that larvae apparently migrate to surface waters at dusk to fill their swimbladders. -

The Freshwater Snails (Mollusca: Gastropoda) of Mexico: Updated Checklist, Endemicity Hotspots, Threats and Conservation Status
Revista Mexicana de Biodiversidad Revista Mexicana de Biodiversidad 91 (2020): e912909 Taxonomy and systematics The freshwater snails (Mollusca: Gastropoda) of Mexico: updated checklist, endemicity hotspots, threats and conservation status Los caracoles dulceacuícolas (Mollusca: Gastropoda) de México: listado actualizado, hotspots de endemicidad, amenazas y estado de conservación Alexander Czaja a, *, Iris Gabriela Meza-Sánchez a, José Luis Estrada-Rodríguez a, Ulises Romero-Méndez a, Jorge Sáenz-Mata a, Verónica Ávila-Rodríguez a, Jorge Luis Becerra-López a, Josué Raymundo Estrada-Arellano a, Gabriel Fernando Cardoza-Martínez a, David Ramiro Aguillón-Gutiérrez a, Diana Gabriela Cordero-Torres a, Alan P. Covich b a Facultad de Ciencias Biológicas, Universidad Juárez del Estado de Durango, Av.Universidad s/n, Fraccionamiento Filadelfia, 35010 Gómez Palacio, Durango, Mexico b Institute of Ecology, Odum School of Ecology, University of Georgia, 140 East Green Street, Athens, GA 30602-2202, USA *Corresponding author: [email protected] (A. Czaja) Received: 14 April 2019; accepted: 6 November 2019 Abstract We present an updated checklist of native Mexican freshwater gastropods with data on their general distribution, hotspots of endemicity, threats, and for the first time, their estimated conservation status. The list contains 193 species, representing 13 families and 61 genera. Of these, 103 species (53.4%) and 12 genera are endemic to Mexico, and 75 species are considered local endemics because of their restricted distribution to very small areas. Using NatureServe Ranking, 9 species (4.7%) are considered possibly or presumably extinct, 40 (20.7%) are critically imperiled, 30 (15.5%) are imperiled, 15 (7.8%) are vulnerable and only 64 (33.2%) are currently stable. -

Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes
Old Dominion University ODU Digital Commons Biological Sciences Theses & Dissertations Biological Sciences Summer 2016 Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes Christi Linardich Old Dominion University, [email protected] Follow this and additional works at: https://digitalcommons.odu.edu/biology_etds Part of the Biodiversity Commons, Biology Commons, Environmental Health and Protection Commons, and the Marine Biology Commons Recommended Citation Linardich, Christi. "Hotspots, Extinction Risk and Conservation Priorities of Greater Caribbean and Gulf of Mexico Marine Bony Shorefishes" (2016). Master of Science (MS), Thesis, Biological Sciences, Old Dominion University, DOI: 10.25777/hydh-jp82 https://digitalcommons.odu.edu/biology_etds/13 This Thesis is brought to you for free and open access by the Biological Sciences at ODU Digital Commons. It has been accepted for inclusion in Biological Sciences Theses & Dissertations by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES by Christi Linardich B.A. December 2006, Florida Gulf Coast University A Thesis Submitted to the Faculty of Old Dominion University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE BIOLOGY OLD DOMINION UNIVERSITY August 2016 Approved by: Kent E. Carpenter (Advisor) Beth Polidoro (Member) Holly Gaff (Member) ABSTRACT HOTSPOTS, EXTINCTION RISK AND CONSERVATION PRIORITIES OF GREATER CARIBBEAN AND GULF OF MEXICO MARINE BONY SHOREFISHES Christi Linardich Old Dominion University, 2016 Advisor: Dr. Kent E. Carpenter Understanding the status of species is important for allocation of resources to redress biodiversity loss. -

PRELIMINARY SURVEY and DIET ANALYSIS of JUVENILE FISHES of an ESTUARINE CREEK on ANDROS ISLAND, BAHAMAS Craig A. Layman and Bria
BULLETIN OF MARINE SCIENCE, 70(l): 199-210, 2002 NOTES PRELIMINARY SURVEY AND DIET ANALYSIS OF JUVENILE FISHES OF AN ESTUARINE CREEK ON ANDROS ISLAND, BAHAMAS CraigA. Layman and Brian R. Silliman Estuarine habitats are important nursery and feeding areas for a variety of fish and invertebrate species. Although numerous studies have investigated trophic linkages in temperate estuarine systems, few have empirically examined these relationships in tropi- cal and subtropical estuaries (Colton and Alevizon, 1983; Heck and Weinstein, 1989; Warburton and Blaber, 1992; Ley et al., 1994; Crabtree et al., 1998). Without knowledge of dietary relationships among organisms, community structure and population interac- tions are difficult to deduce. To this end, a food web approach can be valuable in the study of natural communities (Polis and Winemiller, 1996). Since many tropical and subtropical estuaries are numerically dominated by juvenile fishes (Arrivillaga and Baltz, 1999), the trophic role of these life stages is especially important. Juvenile fish utilization of mangrove and seagrass habitats has been docu- mented in the Caribbean (Robblee and Zieman, 1984; Stoner, 1986; Rooker and Dennis, 1991; Sedberry and Carter, 1993) and Florida (Thayer et al., 1987; Sheridan, 1997; Ley et al., 1999), although few studies have analyzed feeding habitats of the juvenile fishes in these areas (Heck and Weinstein, 1989; Hettler, 1989; Ley et al., 1994). To our knowl- edge, there have been no published studies of the distribution and diet of fishes in estua- rine creeks, and associated seagrass or mangrove areas, in the Bahamian Islands. The purpose of our study was twofold: (1) identify fish species utilizing five major habitat types (sandflat, mangrove, seagrass, rocky structure and artificial structure) of an estuarine creek on Andros Island, Bahamas, and (2) provide a preliminary diet analysis of common juvenile fishes.