Observations on Uric Acid Transport in Man, the Dalmatian and the Non-Dalmatian Dog Howard Duncan
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

2017 Horrible Hundred Report
The Horrible Hundred 2017 A sampling of problem puppy mills and puppy dealers in the United States May 2017 For the fifth straight year, The Humane Society of the United States is reporting on problem puppy mills, including some dealers (re-sellers) and transporters. The Horrible Hundred 2017 report is a list of known, problematic puppy breeding and/or puppy brokering facilities. It is not a list of all puppy mills, nor is it a list of the worst puppy mills in the country. The HSUS provides this update annually, not as a comprehensive inventory, but as an effort to inform the public about common, recurring problems at puppy mills. The information in this report demonstrates the scope of the puppy mill problem in America today, with specific examples of the types of violations that researchers have found at such facilities, for the purposes of warning consumers about the inhumane conditions that so many puppy buyers inadvertently support. The year 2017 has been a difficult one for puppy mill watchdogs. Efforts to get updated information from the United States Department of Agriculture (USDA) on federally-inspected puppy mills were severely crippled due to the USDA’s removal on Feb. 3, 2017 of all animal welfare inspection reports and most enforcement records from the USDA website. As of April 20, 2017, the USDA had restored some Puppies at the facility of Alvin Nolt in Thorpe, Wisconsin, were found on unsafe wire flooring, a repeat violation at the facility. Wire flooring animal welfare records on research facilities and is especially dangerous for puppies because their legs can become other types of dealers, but almost no records on entrapped in the gaps, leaving them unable to reach food, water or pet breeding operations were restored. -

(Dalmatinski Pas) DALMATIAN FCI Group VI
Hrvatski kinološki savez • Croatian Kennel Club DALMATIAN (Dalmatinski pas) DALMATIAN FCI group VI. Standard No. 153 ORIGIN: Croatia DATE OF PUBLICATION OF THE OFFICIAL VALID STANDARD: 13.10.2010. UTILIZATION: Hunting dog. Companion dog, family dog, suitable to be trained for various purposes. FCI-CLASSIFICATION: Group 6 - Scenthounds and related breeds. Section 3 - Related breeds. Without working trials. BRIEF HISTORICAL SUMMARY Breed History he origin of the Dalmatian dog can be recognized in paintings and in church chronicles from the 16th to the 18th century. Dalmatians can be found on the altar painting “Madonna with Jesus and Angels” in the church “Gospa od andjela” in the town Veli Losinj, island Losinj in Croatia, dating back to 1600 – 1630, and also in a fresco in Zaostrog, Dalmatia, Croatia. It suggests that the Dalmatian originates from the eastern Mediterranean region, in particular the historic province Dalmatia. The first descriptions of the Dalmatian were found in the diocese Djakovo, in the Croatia, namely in the church chronicles of the Bishop Petar Bakic from the year 1719 and the church chronicles of Andreas Keczkemety from the year 1737. The dog was named with the Latin name “Canis Dalmaticus” and the height of the dog described with 4-5 “Spithamus”. Thomas Pennant described this breed in his work “Synopsis of Quadrupeds” in the year 1771 as very independent, named it “Dalmatian” and wrote the origin of this breed to be Dalmatia. A work by Thomas Bewick, published in 1790, refers to this breed as “Dalmatian or Coach Dog”. The first unofficial Dalmatian Standard was written by an Englishman named Vero Shaw in the year 1882. -

Uric Acid Metabolism in the Dalmatian Coach Hound
URIC ACID METABOLISM IN THE DALMATIAN COACH HOUND Oliver Martin Briggs Town Thesis submitted in fulfilment of the requirements for the degree of Master of Science (Medicine) in· Chemical Pathology, FacultyCape of Medicine, -University ofof Cape Town. Cape Town, September 1982 University Promoted by Professor E.H. Harley, M.D., Ph.D. The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgementTown of the source. The thesis is to be used for private study or non- commercial research purposes only. Cape Published by the University ofof Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University Ac knowledge men ts I am grate fu 1 to Professor Eric Harley for allowing me to conduct this research while I was engaged in full time practice. His constant enthusiasm, encouragement and guidance was unlimited. Doctors Davidson, Close, Futter and Rowe of St. Francis Veterinary Hospital for assisting in the e x p e r i me n t s . Professor Peter Belonje, Faculty of Medicine, University of Cape Town, for housing experimental subjects. Reckitt & Colman Ltd. and Epol (Pty) Ltd. for their generous assistance in the housing and feeding of the experimental subjects. My wife, Sue, for endlessly typing and retyping this manuscript. TAB LE OF CO NTENTS CHAPTER 1 1. REVIEW 1 1. 1 Introduction 1 1.2 The Initial Report 3 1. 3 The Mode of Inheritance 3 1.4 The Renal and Hepatic Handling of Uric Acid 3 1. -

One Dog's Meat Is Another Dog's Poison—Nutrition in the Dalmatian
123 One dog’s meat is another dog’s poison—nutrition in the Dalmatian dog W.Y. Brown1, B.A. Vanselow2 and S.W. Walkden–Brown1 1School of Rural Science and Agriculture, Animal Science, University of New England, Armidale NSW 2351 2NSW Agriculture Beef Industry Centre, University of New England, Armidale NSW 2351 [email protected] Summary Dalmatian dogs have a predisposition to urate RNA and DNA. Nucleotides resulting from degradation urolithiasis due to a metabolic defect inherent in this of nucleic acids normally undergo hydrolysis to breed. It has been suggested that feeding lowprotein eventually yield free purines and pyrimidines. If not diets can reduce the risk of producing urate calculi in salvaged and reused, these are then degraded further, these dogs, but caution must be exercised to ensure that and the end products excreted (Lehninger 1970). In the adequate nutrient requirements are met. This paper majority of dog breeds, this end product is allantoin. In discusses the results of a recent feeding trial in which the Dalmatian dog however, the conversion of uric acid Dalmatian dogs were fed one of four commercial diets, to allantoin occurs with reduced efficiency (Briggs and or an antiuric acid (vegetarian) diet, or an allmeat Harley 1986) resulting in the excretion of high levels diet. The crude protein content of these diets ranged of uric acid in the urine. from 10.4% to 62.5% in dry matter. Dalmatian dogs It is well documented that the administration of produced significantly lower amounts of precipitates purines to Dalmatian dogs, both orally and in their urine when fed lowprotein diets than when they intravenously, results in corresponding increases in uric were fed premium highprotein diets or meat. -

Mitochondrial Variability of Small Međimurje Dog
ORIGINAL SCIENTIFIC PAPER | 203 Mitochondrial variability of Small Međimurje dog Mateja JANEŠ1 Vladimir BRAJKOVIC1 Ivana DRZAIC1 Minja ZORC2 Peter DOVČ2 Vlatka CUBRIC-CURIK1 (✉) Summary There are six native dog breeds in Croatia recognized by the Federation Cynologique International (FCI) and one national dog breed Small Međimurje dog (MEDI) still unrecognized by the FCI. To promote breed we have analysed mitochondrial DNA control region (CR-mtDNA) sequence (551-bp) in 35 Small Međimurje dogs sampled in Međimurje County. After comparison with 33 worldwide distributed dog breeds (N=115 samples), three main canine CR-mtDNA haplogroups (A, B and C) were observed in Small Međimurje dogs. Median-joining tree showed that MEDI forms six haplotypes presented in haplogroup C (H3 is the most frequent in MEDI population), haplogroup A (haplotypes H2, H5 and H7) and in haplogroup B (haplotypes H4 and H6). The results presented in this study correspond to other mtDNA studies of native dog breeds. For the better genetic description of MEDI and for the optimal future breeding management, further analyses of nuclear genome are recommended. Key words Small Međimurje dog, mitochondrial DNA, haplotype diversity 1 University of Zagreb, Faculty of Agriculture, Department of Animal Science, Zagreb, Croatia 2 University of Ljubljana, Biotechnical Faculty, Department of Animal Science, Ljubljana, Slovenia ✉ Corresponding author: [email protected] Received: May 2, 2019 | Accepted: May 6, 2019 aCS Agric. conspec. sci. Vol. 84 (2019) No. 2 (203-209) 204 | Mateja JANEŠ, Vladimir BRAJKOVIC, Ivana DRZAIC, Minja ZORC, Peter DOVČ, Vlatka CUBRIC-CURIK Introduction Kanthaswamy, 2011) due to its high variability. Canine control Fédération Cynologique Internationale (FCI) recognizes region (CR) of mtDNA is approximately 1270 bp long (Kim et six Croatian native dog breeds: Dalmatian (DALM), Croatian al., 1998). -

Breeds of Cats and Dogs
Breeds of Dogs and Cats Floron C. Faries, Jr. DVM, MS Objectives Discuss the evolution of man’s relationship with dogs and cats Describe the characteristics shared by members of the Canidae family Describe the classification system for dogs List the uses for different breeds of dogs Identify and describe the different breeds of dogs Describe the characteristics shared by members of the family Felidae Describe the classification system for cats Identify and describe the different cat breeds History of Dogs In family Canidae Direct descendents of the wolf Wolf’s scientific name – Canis lupus Dog’s scientific name – Canis familiaris Domestication a few 1,000 years Greece Herding dogs Guarding dogs Hunting dogs Egypt Dogs used in war Bred based on purpose Climate Environment Master’s preference – herding, guarding, hunting 72 million dogs live in U.S. One dog per household in half American family homes More than 228 pure breeds More than 100 mixed breeds Stimulate income of dog industries $11 billion annual sales of dog food Accessory manufacturers Veterinarians Pharmaceutical industry Breeders Racers Trainers Herders Hunters Serve humans Protection Sight Hearing Security Companionship Characteristics of Dogs Size Height 6 inches to 40 inches at the shoulder Life expectancy 9 to 15 years, some 20 years Small dogs live longer than large dogs Common traits Shed hair once a year Non-retractable claws 42 adult teeth Pointed canine teeth Sweating Sweat glands on nose and feet Panting Hearing -

Skillathon Stations: Book 1, Page 12 (Photos from AKC Website) Breed Key
Skillathon stations: Book 1, page 12 (photos from AKC website) Breed Key Terrier Group 1 17 33 Airedale Terrier Parson Russell Terrier Bull Terrier 25 Skye Terrier 16 40 Lakeland Terrier Norwich Terrier Herding Group: Briard 2 Collie 18 34 Shetland Sheepdog 26 Old English 41 German Sheepdog Shepherd 15 Welsh Corgi Working Group: 35 Great Dane 3 Mastiff 19 Standard Schnauzer 27 14 42 Rottweiler German Pinscher Samoyed Toy Group: 48 Chihuahua 4 Maltese 20 Toy Fox Terrier 28 43 13 Pug Toy Poodle Papillon Hound Group: 5 21 36 Bloodhound Borzoi Norwegian Elkhound 29 44 12 Dachshund Whippet Greyhound Miscellaneous Group: 8 24 39 Treeing Walker Coonhound Russell Terrier Chinook 32 47 9 American English Coonhound Norwegian Lundhund Rat Terrier Sporting Group: 6 22 37 English Setter Gordon Setter Weimaraner 30 45 11 Pointer Sussex Spaniel German Shorthaired Pointer Non-Sporting Group: Bichon Frise Dalmatian Chow Chow 7 38 Terrier Working 23 Toy Non-sporting 10 Lhasa Apso Standard Poodle 46 Boston Terrier 31 1. 2. 3. 4. 1a. 2a. 3a. 4a. Senior Senior Senior Senior Herding Hounds Sporting Miscellaneous 5. 6. 7. 8. Senior Senior Senior Senior 5a. 6a. 7a. 8a. Senior Category: Choose a photo of a dog breed. A. Match one dog photo to the correct Dog Breed Group, write the number of the dog photo and B. Identify the dog breed by name in the space below. DOG SKILLATHON - BREED IDENTIFICATION Name: ___________________________Entry #: ___________ Armband: _________ County: ________________________ Team: ____________________ Junior Category Match a photo of dog to correct breed. A. Choose one breed of dog for each Dog Breed Group. -
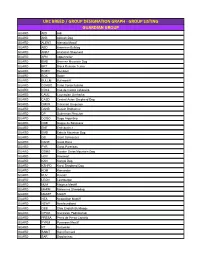
Ukc Breed / Group Designation Graph
UKC BREED / GROUP DESIGNATION GRAPH - GROUP LISTING GUARDIAN GROUP GUARD AIDI Aidi GUARD AKB Akbash Dog GUARD ALENT Alentejo Mastiff GUARD ABD American Bulldog GUARD ANAT Anatolian Shepherd GUARD APN Appenzeller GUARD BMD Bernese Mountain Dog GUARD BRT Black Russian Terrier GUARD BOER Boerboel GUARD BOX Boxer GUARD BULLM Bullmastiff GUARD CORSO Cane Corso Italiano GUARD CDCL Cao de Castro Laboreiro GUARD CAUC Caucasian Ovcharka GUARD CASD Central Asian Shepherd Dog GUARD CMUR Cimarron Uruguayo GUARD DANB Danish Broholmer GUARD DP Doberman Pinscher GUARD DOGO Dogo Argentino GUARD DDB Dogue de Bordeaux GUARD ENT Entlebucher GUARD EMD Estrela Mountain Dog GUARD GS Giant Schnauzer GUARD DANE Great Dane GUARD PYR Great Pyrenees GUARD GSMD Greater Swiss Mountain Dog GUARD HOV Hovawart GUARD KAN Kangal Dog GUARD KSHPD Karst Shepherd Dog GUARD KOM Komondor GUARD KUV Kuvasz GUARD LEON Leonberger GUARD MJM Majorca Mastiff GUARD MARM Maremma Sheepdog GUARD MASTF Mastiff GUARD NEA Neapolitan Mastiff GUARD NEWF Newfoundland GUARD OEB Olde English Bulldogge GUARD OPOD Owczarek Podhalanski GUARD PRESA Perro de Presa Canario GUARD PYRM Pyrenean Mastiff GUARD RT Rottweiler GUARD SAINT Saint Bernard GUARD SAR Sarplaninac GUARD SC Slovak Cuvac GUARD SMAST Spanish Mastiff GUARD SSCH Standard Schnauzer GUARD TM Tibetan Mastiff GUARD TJAK Tornjak GUARD TOSA Tosa Ken SCENTHOUND GROUP SCENT AD Alpine Dachsbracke SCENT B&T American Black & Tan Coonhound SCENT AF American Foxhound SCENT ALH American Leopard Hound SCENT AFVP Anglo-Francais de Petite Venerie SCENT -

AUSTRALIAN NATIONAL KENNEL COUNCIL LTD Judge
AUSTRALIAN NATIONAL KENNEL COUNCIL LTD GROUP 1 - TOYS Judge: Affenpinscher Bourbonnais Pointing Dog * Dachshund (Min. Long) Australian Silky Terrier Bracco Italiano Dachshund (Rabbit Long Bichon Frise Brittany Haired) * Bolognese * Chesapeake Bay Retriever Dachshund (Smooth) Cavalier King Charles Spaniel Clumber Spaniel Dachshund (Min. Smooth) Chihuahua (Long Coat) Cocker Spaniel Dachshund (Rabbit Smooth Chihuahua (Smooth Coat) Cocker Spaniel (American) Haired) * Chinese Crested Dog Curly Coated Retriever Dachshund (Wire) Continental Toy Spaniel Deutch Stichelhaar * Dachshund (Min. Wire) (Papillon) Drentsche Partridge Dog * Dachshund (Rabbit Wire Continental Toy Spaniel English Setter Haired) * (Phalene) * English Springer Spaniel Deerhound Coton De Tulear Field Spaniel Drever * Griffon Belge * Flat Coated Retriever Fawn Brittany Griffon * English Toy Terrier (Black & French Pointing Dog Gascogne Finnish Hound * Tan) Type * Finnish Spitz Griffon Bruxellois French Pointing Dog Pyrenean Foxhound Havanese Type * Gascon Saintongeois * Italian Greyhound French Spaniel * German Hound * Japanese Chin French Water Dog * Grand Basset Griffon King Charles Spaniel Frisian Pointing Dog * Vendeen Kromforhlander * Frisian Water Dog * Grand Griffon Vendeen * Lowchen German Shorthaired Pointer Great Anglo French-White & Maltese German Wirehaired Pointer Black Hound * Miniature Pinscher German Spaniel * Great Anglo-French Tri Colour Hound * Pekingese Golden Retriever Great Anglo-French White & Petit Brabancon * Gordon Setter Orange Hound * Pomeranian -

101 Dalmatians KIDS (Ages 6-11; All Youth
101 Dalmatians KIDS (ages 6-11; all youth are cast) Disney’s classic animated tale of kidnapping villains and courageous puppies is adapted in this "fur-tastic" musical adventure. Based on the classic animated film, Disney's 101 Dalmatians KIDS is a fur-raising adventure featuring Cruella De Vil, Disney's most outrageous villain, and 101 of the most adorable heroes to set their paws onstage. With a high-spirited score and lovable characters, this stage adaptation is certain to charm and delight all audiences. Pet owners, Roger and Anita, live happily in London with their Dalmatians, Pongo and Perdita, stalwart dogs devoted to raising their puppies. Everything is quiet until Anita's former classmate, the monstrous Cruella De Vil, plots to steal the puppies for her new fur coat. The Dalmatians rally all the dogs of London for a daring rescue of the puppies from Cruella and her bumbling henchmen. Cruella De Vil Cruella is the rich, fashion-forward antagonist of the story who harbors a penchant for puppy fur. Ruthless and impulsive, she should command the stage with dramatic flair and brassiness. Strong singer and actress with an authoritative presence and can move with a high, captivating energy. Gender: Female Vocal range top: C4 Vocal range bottom: Ab3 Roger Roger, married to Anita, is the soft-spoken yet confident composer and owner of Pongo and Perdita. His relaxed form of leadership acts as a contrast to the frenetic Cruella. He should be a strong actor, singer, and mover with an adult bearing that sets him apart from the more immature puppies. -

Breed Specific Instructions (BSI) Regarding Exaggerations in Pedigree Dogs
By the Nordic Kennel Clubs 2014 Applicable from 2014 Breed Specific Instructions (BSI) regarding exaggerations in pedigree dogs DANSK KENNEL KLUB HUNDARÆKTARFÉLAG ÍSLANDS NORSK KENNEL KLUB SUOMEN KENNELLIITTO SVENSKA KENNELKLUBBEN Index Introduction .............................................................................................................................4 Application ..............................................................................................................................6 Basics for all dogs ...................................................................................................................8 Breed types ...........................................................................................................................10 FCI GROUP 1 Sheepdogs and Cattle Dogs ..............................................................................12 FCI GROUP 2 Pinscher and Schnauzer - Molossoïd Breeds - Swiss Mountain and Cattle Dogs ...14 FCI GROUP 3 Terriers .............................................................................................................19 FCI GROUP 4 Dachshunds ......................................................................................................21 FCI GROUP 5 Spitz and Primitive types ...................................................................................22 FCI GROUP 6 Scenthounds and Related Breeds ......................................................................24 FCI GROUP 7 Pointing Dogs ....................................................................................................26 -

DUSTY RHODES—Hamilton County Auditor 2021 DOG & KENNEL
DUSTY RHODES—Hamilton County Auditor 2021 DOG & KENNEL Please select the breed which comes closest to describing your pet. If your pet is a combination of breeds, please choose the Breeds most recognizable breed, use that breed, followed by the letter “M” (for mixed breed). Your accuracy helps us in our efforts to reunite lost dogs with their owners. Listed below are Breed Names: Affenpinscher Brittany Spaniel French Bulldog Mastiff Scottish Terrier Afghan Hound Brussels Griffon German Pinscher Miniature Pinscher Sealyham Terrier Airedale Terrier Bull Terrier German Shepherd; Shepherd Mountain Cur Shar-Pei Akbash Dog Bulldog German Shorthaired Pointer Neapolitan Mastiff Shetland Sheepdog, Sheltie, Toy Collie Akita Bullmastiff German Wirehaired Pointer Newfoundland Shiba Inu Alaskan Malamute; Malamute Cairn Terrier Glen of Imal Terrier Norfolk Terrier Shih Tzu American Bulldog Canaan Dog Golden Retriever Norwegian Buhund Siberian Husky, Husky American Eskimo; Spitz Cane Corso Gordon Setter Norwegian Elkhound Silky Terrier American Pit Bull Terrier Catahoula Leopard Dog Great Dane Norwich Terrier Skye Terrier American Staffordshire Cavalier King Charles Spaniel Great Pyrenees Nova Scotia Duck Tolling Soft Coated Wheaten Terrier Terrier Retriever American Water Spaniel Cesky Terrier Greater Swiss Mountain Dog Old English Sheepdog Springer Spaniel Anatolian Shepherd Chesapeake Bay Retriever Greyhound Otterhound Staffordshire Bull Terrier Australian Cattle Dog Chihuahua Harrier Papillon Sussex Spaniel Australian Kelpie Chinese Crested Havanese