H2-Driven CO2 Fixation by Extremely Thermoacidophilic Archaea
Total Page:16
File Type:pdf, Size:1020Kb
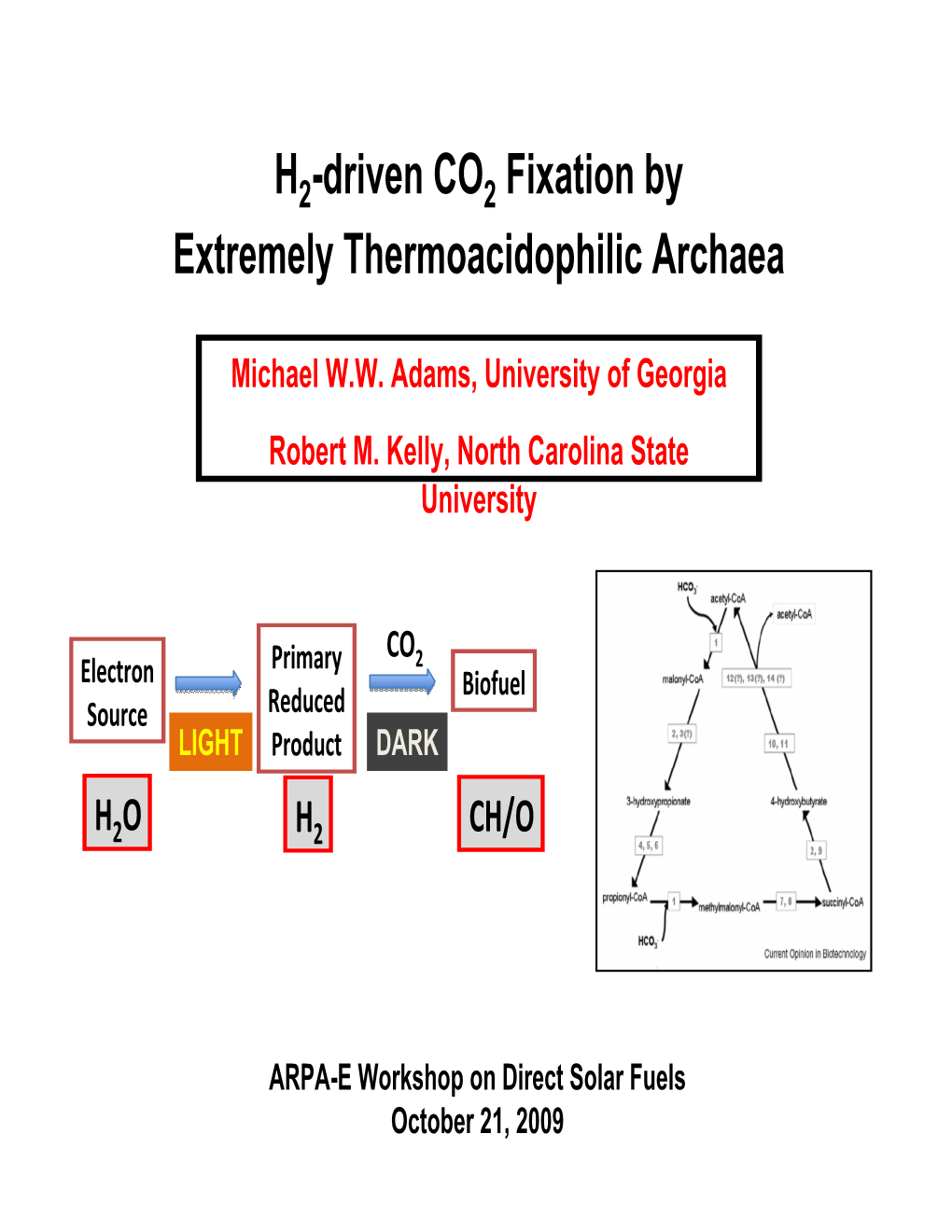
Load more
Recommended publications
-

Proteome Cold-Shock Response in the Extremely Acidophilic Archaeon, Cuniculiplasma Divulgatum
microorganisms Article Proteome Cold-Shock Response in the Extremely Acidophilic Archaeon, Cuniculiplasma divulgatum Rafael Bargiela 1 , Karin Lanthaler 1,2, Colin M. Potter 1,2 , Manuel Ferrer 3 , Alexander F. Yakunin 1,2, Bela Paizs 1,2, Peter N. Golyshin 1,2 and Olga V. Golyshina 1,2,* 1 School of Natural Sciences, Bangor University, Deiniol Rd, Bangor LL57 2UW, UK; [email protected] (R.B.); [email protected] (K.L.); [email protected] (C.M.P.); [email protected] (A.F.Y.); [email protected] (B.P.); [email protected] (P.N.G.) 2 Centre for Environmental Biotechnology, Bangor University, Deiniol Rd, Bangor LL57 2UW, UK 3 Systems Biotechnology Group, Department of Applied Biocatalysis, CSIC—Institute of Catalysis, Marie Curie 2, 28049 Madrid, Spain; [email protected] * Correspondence: [email protected]; Tel.: +44-1248-388607; Fax: +44-1248-382569 Received: 27 April 2020; Accepted: 15 May 2020; Published: 19 May 2020 Abstract: The archaeon Cuniculiplasma divulgatum is ubiquitous in acidic environments with low-to-moderate temperatures. However, molecular mechanisms underlying its ability to thrive at lower temperatures remain unexplored. Using mass spectrometry (MS)-based proteomics, we analysed the effect of short-term (3 h) exposure to cold. The C. divulgatum genome encodes 2016 protein-coding genes, from which 819 proteins were identified in the cells grown under optimal conditions. In line with the peptidolytic lifestyle of C. divulgatum, its intracellular proteome revealed the abundance of proteases, ABC transporters and cytochrome C oxidase. From 747 quantifiable polypeptides, the levels of 582 proteins showed no change after the cold shock, whereas 104 proteins were upregulated suggesting that they might be contributing to cold adaptation. -

Microbial Desulfurization of Three Different Coals from Indonesia, China and Korea in Varying Growth Medium
Korean J. Chem. Eng., 30(3), 680-687 (2013) DOI: 10.1007/s11814-012-0168-z INVITED REVIEW PAPER Microbial desulfurization of three different coals from Indonesia, China and Korea in varying growth medium Dong-Jin Kim*, Chandra Sekhar Gahan*,**,†, Chandrika Akilan***, Seo-Yun Choi*, and Byoung-Gon Kim* *Mineral Resource Research Division, Korea Institute of Geoscience and Mineral Resources (KIGAM), Gwahang-ro 92, Yuseong-gu, Daejeon 305-350, Korea **SRM Research Institute, SRM University, Kattankulathur - 603 203, Kancheepuram District, Chennai, Tamil Nadu, India ***Faculty of Minerals and Energy, School of Chemical and Mathematical Sciences, Murdoch University, 90 South Street, Murdoch, 6150, Western Australia (Received 23 April 2012 • accepted 2 October 2012) Abstract−Shake flask studies on microbial desulfurization of three different coal samples (Indonesian lignite, Chinese lignite and Korean anthracite) were performed to optimize the best suitable growth medium. Among the three different growth mediums (basal salt medium, basal salt medium supplemented with 9 g/L Fe and basal salt medium supple- mented with 2.5% S0) tested, the basal salt medium was found to be the best, considering process dynamics and eco- nomical factors. The extent of pyrite oxidation was highest with 95% in the experiments with Korean anthracite in basal salt medium supplemented with 9 g/L Fe, while the lowest pyrite oxidation of 70-71% was observed in the experiments with Indonesian and Chinese Lignite’s in only basal salt medium. The microbial sulfur removal in the experiments with basal salt medium supplemented with 9 g/L Fe for all the three coal samples was between 94-97%, while the experiments on basal salt medium supplemented with 2.5% S0 for all the coal samples were relatively much lower ranging between 27-48%. -

Resolution of Carbon Metabolism and Sulfur-Oxidation Pathways of Metallosphaera Cuprina Ar-4 Via Comparative Proteomics
JOURNAL OF PROTEOMICS 109 (2014) 276– 289 Available online at www.sciencedirect.com ScienceDirect www.elsevier.com/locate/jprot Resolution of carbon metabolism and sulfur-oxidation pathways of Metallosphaera cuprina Ar-4 via comparative proteomics Cheng-Ying Jianga, Li-Jun Liua, Xu Guoa, Xiao-Yan Youa, Shuang-Jiang Liua,c,⁎, Ansgar Poetschb,⁎⁎ aState Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, Beijing, PR China bPlant Biochemistry, Ruhr University Bochum, Bochum, Germany cEnvrionmental Microbiology and Biotechnology Research Center, Institute of Microbiology, Chinese Academy of Sciences, Beijing, PR China ARTICLE INFO ABSTRACT Article history: Metallosphaera cuprina is able to grow either heterotrophically on organics or autotrophically Received 16 March 2014 on CO2 with reduced sulfur compounds as electron donor. These traits endowed the species Accepted 6 July 2014 desirable for application in biomining. In order to obtain a global overview of physiological Available online 14 July 2014 adaptations on the proteome level, proteomes of cytoplasmic and membrane fractions from cells grown autotrophically on CO2 plus sulfur or heterotrophically on yeast extract Keywords: were compared. 169 proteins were found to change their abundance depending on growth Quantitative proteomics condition. The proteins with increased abundance under autotrophic growth displayed Bioleaching candidate enzymes/proteins of M. cuprina for fixing CO2 through the previously identified Autotrophy 3-hydroxypropionate/4-hydroxybutyrate cycle and for oxidizing elemental sulfur as energy Heterotrophy source. The main enzymes/proteins involved in semi- and non-phosphorylating Entner– Industrial microbiology Doudoroff (ED) pathway and TCA cycle were less abundant under autotrophic growth. Also Extremophile some transporter proteins and proteins of amino acid metabolism changed their abundances, suggesting pivotal roles for growth under the respective conditions. -

A Survey of Carbon Fixation Pathways Through a Quantitative Lens
Journal of Experimental Botany, Vol. 63, No. 6, pp. 2325–2342, 2012 doi:10.1093/jxb/err417 Advance Access publication 26 December, 2011 REVIEW PAPER A survey of carbon fixation pathways through a quantitative lens Arren Bar-Even, Elad Noor and Ron Milo* Department of Plant Sciences, The Weizmann Institute of Science, Rehovot 76100, Israel * To whom correspondence should be addressed. E-mail: [email protected] Received 15 August 2011; Revised 4 November 2011; Accepted 8 November 2011 Downloaded from Abstract While the reductive pentose phosphate cycle is responsible for the fixation of most of the carbon in the biosphere, it http://jxb.oxfordjournals.org/ has several natural substitutes. In fact, due to the characterization of three new carbon fixation pathways in the last decade, the diversity of known metabolic solutions for autotrophic growth has doubled. In this review, the different pathways are analysed and compared according to various criteria, trying to connect each of the different metabolic alternatives to suitable environments or metabolic goals. The different roles of carbon fixation are discussed; in addition to sustaining autotrophic growth it can also be used for energy conservation and as an electron sink for the recycling of reduced electron carriers. Our main focus in this review is on thermodynamic and kinetic aspects, including thermodynamically challenging reactions, the ATP requirement of each pathway, energetic constraints on carbon fixation, and factors that are expected to limit the rate of the pathways. Finally, possible metabolic structures at Weizmann Institute of Science on July 3, 2016 of yet unknown carbon fixation pathways are suggested and discussed. -

Exploring the Microbial Biotransformation of Extraterrestrial
www.nature.com/scientificreports OPEN Exploring the microbial biotransformation of extraterrestrial material on nanometer scale Tetyana Milojevic1*, Denise Kölbl1, Ludovic Ferrière 2, Mihaela Albu 3, Adrienne Kish4, Roberta L. Flemming5, Christian Koeberl2,9, Amir Blazevic1, Ziga Zebec 1,6, Simon K.-M. R. Rittmann 6, Christa Schleper 6, Marc Pignitter7, Veronika Somoza7, Mario P. Schimak8 & Alexandra N. Rupert 5 Exploration of microbial-meteorite redox interactions highlights the possibility of bioprocessing of extraterrestrial metal resources and reveals specifc microbial fngerprints left on extraterrestrial material. In the present study, we provide our observations on a microbial-meteorite nanoscale interface of the metal respiring thermoacidophile Metallosphaera sedula. M. sedula colonizes the stony meteorite Northwest Africa 1172 (NWA 1172; an H5 ordinary chondrite) and releases free soluble metals, with Ni ions as the most solubilized. We show the redox route of Ni ions, originating from the metallic Ni° of the meteorite grains and leading to released soluble Ni2+. Nanoscale resolution ultrastructural studies of meteorite grown M. sedula coupled to electron energy loss spectroscopy (EELS) points to the redox processing of Fe-bearing meteorite material. Our investigations validate the ability of M. sedula to perform the biotransformation of meteorite minerals, unravel microbial fngerprints left on meteorite material, and provide the next step towards an understanding of meteorite biogeochemistry. Our fndings will serve in defning mineralogical and morphological criteria for the identifcation of metal-containing microfossils. Te ability of chemolithotrophic microorganisms to catalyze redox transformations of metals is an exquisite tool for energy transduction between a mineral body and a living entity. Te diferent types of meteorites from diverse parental bodies (asteroids, Moon, or Mars) represent exceptional metal-bearing substrates that have experienced an exposure to multiple extreme conditions during their interstellar or interplanetary travel. -

Microbiology)
Goa University P.O. Goa University, Taleigao Plateau, Goa 403 206, India Syllabus for entrance to Ph.D./M.Phil. (Microbiology) MICROBIAL BIOCHEMISTRY 1. Biological Molecules 1.1 Proteins Amino acids: features and properties. Protein: structure, principles of separation and purification, molecular weight determination; sequencing and synthesis. Enzymes: activity, inhibition, mechanism of action; regulatory – allosteric and covalently modulated enzymes and their significance in metabolism. 1.2 Carbohydrates Monosaccharides: types, characteristics and properties. Disaccharides, oligosaccharides, polysaccharides – biological significance. 1.3 Lipids Fatty acids: saturated and unsaturated, structure and properties. Lipids: biological significance; lipid composition of microorganisms. 2. Bioenergetics and Carbohydrate Metabolism 2.1 Bioenergetics Thermodynamics, exergonic and endergonic reactions, redox potential, high energy compounds, ATP structure and significance. 2.2 Oxidative Phosphorylation Redox enzymes, aerobic electron transport and oxidative phosphorylation. 2.3 Carbohydrate metabolism A. Carbohydrates: Central pathways of metabolism – regulatory mechanisms, bioenergetics and significance – EMP, TCA cycle (glucose aerobic and anaerobic metabolism, malate metabolism), Glyoxylate cycle. B. Gluconeogenesis from TCA intermediates / amino acids / acetyl-CoA; biosynthesis of polysaccharides and sugar interconversions. 3. Lipids, Amino Acids, Nucleotides and other Metabolic Paths 3.1 Lipid Metabolism A. Anabolism: Biosynthesis of fatty -

Planktonic Euryarchaeota Are a Significant Source of Archaeal Tetraether Lipids in the Ocean
Planktonic Euryarchaeota are a significant source of archaeal tetraether lipids in the ocean Sara A. Lincolna,b,1,2, Brenner Waib,c, John M. Eppleyb,d, Matthew J. Churchb,c, Roger E. Summonsa, and Edward F. DeLongb,c,d,2 aDepartment of Earth, Atmospheric and Planetary Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139; bCenter for Microbial Oceanography: Research and Education, University of Hawaii at Manoa, Honolulu, HI 96822; cDepartment of Oceanography, School of Ocean and Earth Science and Technology, University of Hawaii at Manoa, Honolulu, HI 96822; and dDepartment of Civil and Environmental Engineering and Division of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 Contributed by Edward F. DeLong, May 23, 2014 (sent for review October 6, 2013) Archaea are ubiquitous in marine plankton, and fossil forms of Thaumarchaeota (1, 2, 12)—have been isolated in pure culture. archaeal tetraether membrane lipids in sedimentary rocks docu- All MG-I strains isolated to date are chemolithoautotrophic, ment their participation in marine biogeochemical cycles for >100 fixing inorganic carbon via energy obtained from the oxidation of million years. Ribosomal RNA surveys have identified four major ammonia to nitrite (13). Recent evidence suggests that MG-I clades of planktonic archaea but, to date, tetraether lipids have also contribute to the flux of potent greenhouse gases nitrous been characterized in only one, the Marine Group I Thaumarch- oxide (14) and methane (15) from the water column to the at- aeota. The membrane lipid composition of the other planktonic mosphere. The membrane lipid assemblage of MG-I includes — — archaeal groups all uncultured Euryarchaeota is currently un- GDGTs with zero through four cyclopentyl moieties and cren- known. -

ABSTRACT AUERNIK, KATHRYNE SHERLOCK. Functional Genomics Analysis of Metal Mobilization by the Extremely Thermoacidophilic Archa
ABSTRACT AUERNIK, KATHRYNE SHERLOCK. Functional Genomics Analysis of Metal Mobilization by the Extremely Thermoacidophilic Archaeon Metallosphaera sedula. (Under the direction of Dr. Robert Kelly.) Biomining processes recovering base, strategic and precious metals have predominantly utilized mesophilic bacteria, but relatively low yields have impacted wider application of this biotechnology. However, the use of high temperature microorganisms offers great potential to increase metal mobilization rates. Metallosphaera sedula (Mse) is an extremely thermoacidophilic archaeon with bioleaching capabilities, although little is known about the physiology of this microorganism. To better characterize Mse, its genome was sequenced and a whole genome oligonucleotide microarray was constructed for transcriptional response analysis. The physiological and bioenergetic complexities of Mse bioleaching were studied focusing on iron oxidation, sulfur oxidation, and growth modes (heterotrophy, autotrophy, and mixotrophy). The transcriptomes corresponding to each of these elements were examined for clues to the mechanisms by which Mse oxidizes inorganic energy sources (i.e. metal sulfides) and fixes CO2. Quinol/terminal oxidases important for maintaining intracellular pH and contributing to ATP generation via proton pumping were stimulated by different energy sources. The soxABCDD’L genome locus (Msed_0285-Msed_0291) was stimulated in the presence of reduced inorganic sulfur compounds (RISCs) and H2, while the soxNL-CbsABA cluster (Msed_0500-Msed_0504) was induced by Fe(II). Two similar copies of the SoxB/CoxI-like cytochrome oxidase subunit, foxAA’ (Msed_0484/Msed_0485) were implicated in fox cluster oxidation of Fe(II), as well as other energy sources. The doxBCE locus (Msed_2030-Msed2032) did not respond uniformly to either Fe(II) or RISCs, but was up-regulated in the presence of chalcopyrite (CuFeS2). -

Life in Extreme Environments
insight review articles Life in extreme environments Lynn J. Rothschild & Rocco L. Mancinelli NASA Ames Research Center, Moffett Field, California 94035-1000, USA (e-mail: [email protected]; [email protected]) Each recent report of liquid water existing elsewhere in the Solar System has reverberated through the international press and excited the imagination of humankind. Why? Because in the past few decades we have come to realize that where there is liquid water on Earth, virtually no matter what the physical conditions, there is life. What we previously thought of as insurmountable physical and chemical barriers to life, we now see as yet another niche harbouring ‘extremophiles’. This realization, coupled with new data on the survival of microbes in the space environment and modelling of the potential for transfer of life between celestial bodies, suggests that life could be more common than previously thought. Here we examine critically what it means to be an extremophile, and the implications of this for evolution, biotechnology and especially the search for life in the Universe. ormal is passé; extreme is chic. While thriving in biological extremes (for example, nutritional Aristotle cautioned “everything in extremes, and extremes of population density, parasites, moderation”, the Romans, known for their prey, and so on). excesses, coined the word ‘extremus’, the ‘Extremophile’ conjures up images of prokaryotes, yet the superlative of exter (‘being on the outside’). taxonomic range spans all three domains. Although all NBy the fifteenth century ‘extreme’ had arrived, via Middle hyperthermophiles are members of the Archaea and French, to English. At the dawning of the twenty-first Bacteria, eukaryotes are common among the psychrophiles, century we know that the Solar System, and even Earth, acidophiles, alkaliphiles, piezophiles, xerophiles and contain environmental extremes unimaginable to the halophiles (which respectively thrive at low temperatures, low ‘ancients’ of the nineteenth century. -

Extremophiles-Basic Concepts
CONTENTS CONTENTS EXTREMOPHILES Extremophiles - Volume 1 No. of Pages: 396 ISBN: 978-1-905839-93-3 (eBook) ISBN: 978-1-84826-993-4 (Print Volume) Extremophiles - Volume 2 No. of Pages: 392 ISBN: 978-1-905839-94-0 (eBook) ISBN: 978-1-84826-994-1 (Print Volume) Extremophiles - Volume 3 No. of Pages: 364 ISBN: 978-1-905839-95-7 (eBook) ISBN: 978-1-84826-995-8 (Print Volume) For more information of e-book and Print Volume(s) order, please click here Or contact : [email protected] ©Encyclopedia of Life Support Systems (EOLSS) EXTREMOPHILES CONTENTS VOLUME I Extremophiles: Basic Concepts 1 Charles Gerday, Laboratory of Biochemistry, University of Liège, Belgium 1. Introduction 2. Effects of Extreme Conditions on Cellular Components 2.1. Membrane Structure 2.2. Nucleic Acids 2.2.1. Introduction 2.2.2. Desoxyribonucleic Acids 2.2.3. Ribonucleic Acids 2.3. Proteins 2.3.1. Introduction 2.3.2. Thermophilic Proteins 2.3.2.1. Enthalpically Driven Stabilization Factors: 2.3.2.2. Entropically Driven Stabilization Factors: 2.3.3. Psychrophilic Proteins 2.3.4. Halophilic Proteins 2.3.5. Piezophilic Proteins 2.3.5.1. Interaction with Other Proteins and Ligands: 2.3.5.2. Substrate Binding and Catalytic Efficiency: 2.3.6. Alkaliphilic Proteins 2.3.7. Acidophilic Proteins 3. Conclusions Extremophiles: Overview of the Biotopes 43 Michael Gross, University of London, London, UK 1. Introduction 2. Extreme Temperatures 2.1. Terrestrial Hot Springs 2.2. Hot Springs on the Ocean Floor and Black Smokers 2.3. Life at Low Temperatures 3. High Pressure 3.1. -

4 Metabolic and Taxonomic Diversification in Continental Magmatic Hydrothermal Systems
Maximiliano J. Amenabar, Matthew R. Urschel, and Eric S. Boyd 4 Metabolic and taxonomic diversification in continental magmatic hydrothermal systems 4.1 Introduction Hydrothermal systems integrate geological processes from the deep crust to the Earth’s surface yielding an extensive array of spring types with an extraordinary diversity of geochemical compositions. Such geochemical diversity selects for unique metabolic properties expressed through novel enzymes and functional characteristics that are tailored to the specific conditions of their local environment. This dynamic interaction between geochemical variation and biology has played out over evolu- tionary time to engender tightly coupled and efficient biogeochemical cycles. The timescales by which these evolutionary events took place, however, are typically in- accessible for direct observation. This inaccessibility impedes experimentation aimed at understanding the causative principles of linked biological and geological change unless alternative approaches are used. A successful approach that is commonly used in geological studies involves comparative analysis of spatial variations to test ideas about temporal changes that occur over inaccessible (i.e. geological) timescales. The same approach can be used to examine the links between biology and environment with the aim of reconstructing the sequence of evolutionary events that resulted in the diversity of organisms that inhabit modern day hydrothermal environments and the mechanisms by which this sequence of events occurred. By combining molecu- lar biological and geochemical analyses with robust phylogenetic frameworks using approaches commonly referred to as phylogenetic ecology [1, 2], it is now possible to take advantage of variation within the present – the distribution of biodiversity and metabolic strategies across geochemical gradients – to recognize the extent of diversity and the reasons that it exists. -

The Distribution and Evolution of Small Non-Coding Rnas in Archaea in Light of New Archaeal Phyla
THE DISTRIBUTION AND EVOLUTION OF SMALL NON-CODING RNAS IN ARCHAEA IN LIGHT OF NEW ARCHAEAL PHYLA A thesis submitted in partial fulfilment of the requirements for the Degree of Master of Science in Biological Sciences at the University of Canterbury by Laura A.A. Grundy University of Canterbury 2016 Table of Contents Table of Contents...................................................................................................... 2 Acknowledgements ................................................................................................... 4 Abstract..................................................................................................................... 5 Chapter One - Introduction ..................................................................................... 6 Overview ............................................................................................................... 6 The Archaeal Domain of Life ................................................................................. 6 Metagenomics and the Availability of Genomic Data ......................................... 6 The History of Archaeal Taxa ............................................................................ 7 Archaeal Similarities to the Bacteria and Eukaryotes......................................... 9 Small Non-Coding RNA....................................................................................... 10 RNA................................................................................................................